All published articles of this journal are available on ScienceDirect.
Navigating Dental Care and Oral Health Management Challenges in Sjögren's Syndrome: A Comprehensive Case Report with Literature Review
Abstract
Background
Autoimmune diseases encompass a wide range of conditions in which the immune system erroneously targets the body's own cells, leading to inflammation and tissue damage. Among these disorders, Sjögren’s syndrome holds particular significance in dentistry. This chronic condition primarily impacts exocrine glands, causing hallmark symptoms, such as xerostomia and keratoconjunctivitis sicca. Current research underscores the pivotal role of lymphocyte infiltration, particularly by T and B cells, in driving glandular dysfunction. Diagnostic markers, such as anti-SSA/Ro and anti-SSB/La autoantibodies, play a crucial role in confirming the condition. Furthermore, disruptions in microbial balance, or dysbiosis, are believed to exacerbate immune system dysregulation, further contributing to disease progression. This study presented the case of a 47-year-old female who has been diagnosed with Sjögren’s disease. Additionally, her medical history included complications from Stevens-Johnson syndrome, a stroke, trachoma, vitiligo, hypertension, and pre-diabetes. The objective of this study was to provide a detailed overview of the patient’s symptoms and treatment approach, emphasizing the impact of Sjögren’s syndrome on oral health and systemic complications.
Case Report
This case study examined a 47-year-old female patient diagnosed with Sjögren’s syndrome, a chronic autoimmune condition characterized by the dysfunction of exocrine glands. Additionally, she presented many other medical conditions, which complicated her management. The patient presented with severe xerostomia and recurrent oral infections resulting from diminished salivary function. Laboratory findings indicated renal decline and elevated inflammatory markers, while a biopsy confirmed the diagnosis through lymphoid aggregates. This case underscores the multifaceted challenges in managing Sjögren’s syndrome, especially when accompanied by systemic involvement and multiple co-morbidities. The interplay between the autoimmune mechanisms of Sjögren’s syndrome and other conditions, such as Stevens-Johnson syndrome and vitiligo, creates a complex clinical scenario. Patients with Sjögren’s syndrome are at an increased risk for complications, including renal impairment, neurological issues, and a higher incidence of malignancies like non-Hodgkin’s lymphoma. Effective management requires a multidisciplinary approach, integrating specialists from various fields. Symptom relief is paramount, particularly for oral manifestations, as xerostomia can significantly impair quality of life. The patient's treatment included hydroxychloroquine to manage autoimmune responses and pilocarpine to stimulate saliva production. Regular monitoring of renal and liver functions, as well as inflammatory markers, is essential to prevent deterioration. Moreover, the presence of systemic inflammation, as indicated by elevated CRP levels, necessitates careful consideration of medication management to avoid exacerbating existing conditions. A comprehensive treatment strategy that addresses both the autoimmune components and the patient's broader health concerns is crucial for optimizing outcomes.
Conclusion
This case highlights the importance of individualized care, early detection, and comprehensive monitoring in patients with Sjögren’s syndrome. A comprehensive approach that accounts for the complexities of multiple co-morbidities can significantly improve quality of life and prevent further complications.
1. INTRODUCTION
Autoimmune diseases have long been recognized as a diverse group of conditions that attack the body's own cells, leading to inflammation and damage. They can occur almost anywhere in the body, and 80 such conditions have been recognized so far [1]. One such disorder of peculiar importance for dentistry is Sjögren’s syndrome. It is a chronic autoimmune condition marked by the deterioration of the body's exocrine glands, resulting in distinctive symptoms, such as xerostomia and keratoconjunctivitis sicca. Recent developments have pointed towards the role of lymphocyte infiltration, particularly T and B cells, in glandular inflammation and dysfunction. The presence of auto-antibodies, such as anti-SSA/Ro and anti-SSB/La, is commonly associated with the condition and are important biomarkers for diagnosis [2]. Moreover, the relationship between the microbiome and autoimmune mechanisms has garnered significant attention, suggesting that microbial dysbiosis may exacerbate immune responses [3]. Nevertheless, Sjögren's syndrome has a broader impact than just causing symptoms in the mouth and eyes. It also affects many organ systems, such as the kidneys, liver, and hematologic systems, making diagnosis and treatment more complex.
This study aimed to investigate the case of a 47-year-old female who has been diagnosed with Sjögren’s disease. Additionally, her medical history included complications from Stevens-Johnson syndrome, a stroke, trachoma, vitiligo, hypertension, and prediabetes. Apart from typical symptoms of dry eyes and mouth, the patient has had a nearly total impairment of salivary gland function, resulting in notable oral health difficulties, such as swallowing problems and increased susceptibility to dental decay and oral infections. The examination of her salivary glands through a biopsy showed the presence of lymphoid aggregates, which provided additional evidence supporting the diagnosis of Sjögren’s syndrome. The patient's intricate medical background presents challenges that require a comprehensive approach to her treatment, which involves several fields in healthcare. The objective of this study was to present a detailed case study of a 47-year-old female patient with Sjögren’s syndrome, highlighting its impact on her kidneys and liver, along with the associated intraoral and extraoral symptoms. The patient's intricate medical background, which encompassed Stevens-Johnson syndrome, stroke, trachoma, vitiligo, hypertension, and pre-diabetes, added more complexity to the care of her condition. Nevertheless, a careful approach and meticulous designing of the regimen ensured optimum outcomes. The findings of this study shed light on the overall approach to a patient with Sjögren’s syndrome seeking dental treatment.
2. CASE REPORT
A 47-year-old female patient reported to the College of Dentistry, Jouf University, Saudi Arabia, with significant oral discomfort, including difficulty in swallowing, a persistent dry mouth, and recurrent oral infections, likely due to the severely diminished function of her salivary glands, presenting with associated dryness of the mouth and eyes (a common feature of Sjögren’s syndrome) (Fig. 1). She has been experiencing these symptoms since 2019. The patient was also suffering from trachoma and, subsequently, vitiligo for 17 years. In addition, with typical presentations of Sjögren’s disease, she also presented with several other medical issues that had notable clinical significance. She reported in ER 14 years ago with an episode of Stevens-Johnson syndrome with recurrence again 10 years later. Following that, she experienced an episode of stroke in 2021. During this time, she was on oral contraceptive medications for a prolonged period. She reported a recent history of hypertension and diabetes mellitus type 2 and was currently under medication for the same from her primary physician.
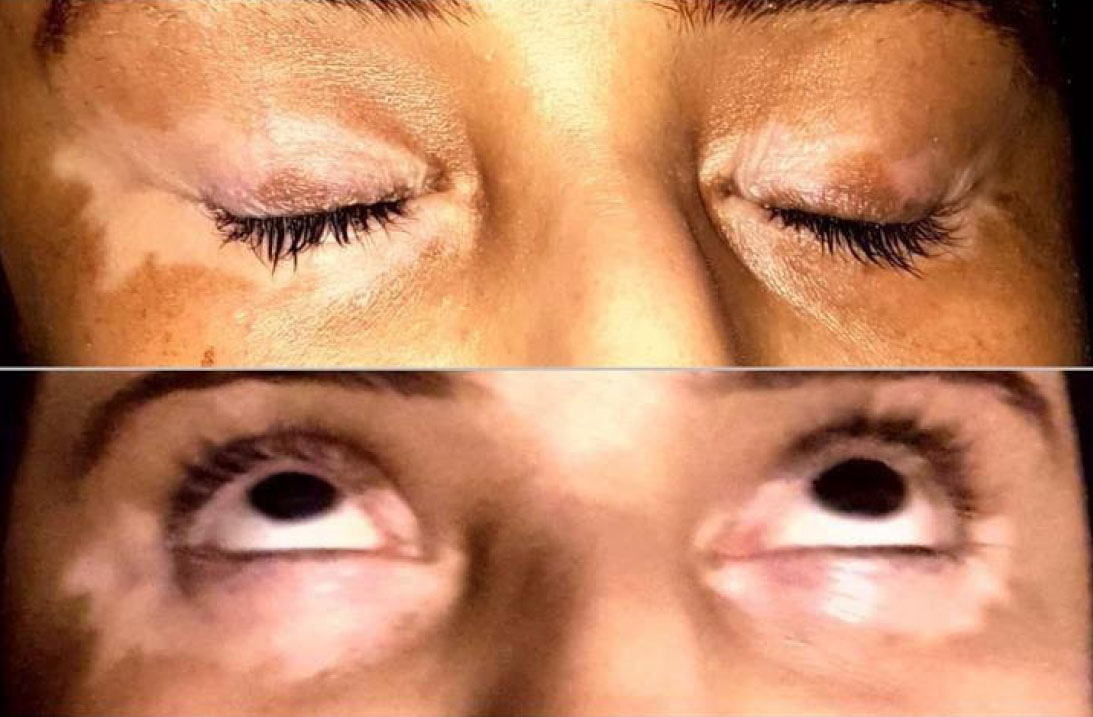
Patient’s eyes showing signs of dryness associated with Sjögren’s syndrome.
Table 1 presents the case history and diagnosis workflow in the management of this patient.
| Patient Presentation | A 47-year-old female presented with oral discomfort, difficulty swallowing, persistent dry mouth, and recurrent oral infections. Symptoms have been present since 2019. | Severe xerostomia and dryness of mouth and eyes consistent with Sjögren’s syndrome. |
| Medical History | History of Stevens-Johnson syndrome (14 and 10 years ago), stroke (2021), prolonged use of oral contraceptives, trachoma, vitiligo, hypertension, and type 2 diabetes. | Systemic complications, including dermatological (hyperpigmentation, nail fissuring) and vascular (stroke, hypertension) issues. |
| Clinical Examination | Hyperpigmentation and nail fissuring linked to Stevens-Johnson syndrome (Figs. 2 and 3). | Evidence of epidermal damage and matrix involvement during Stevens-Johnson syndrome episodes. |
| Management Approach | Multidisciplinary care involving hydroxychloroquine (immune management) and pilocarpine (saliva stimulation). | Symptom relief with sugar-free gum and eye lubricants; careful drug selection to avoid Stevens-Johnson syndrome triggers. |
| Monitoring | Regular assessment of renal and liver function and systemic inflammatory markers. | Essential to prevent the progression of systemic complications and adjust treatment strategies. |
Fig. (2) shows the hyperpigmentation changes associated with Stevens-Johnson syndrome. The patient's history, clinical presentation, and timing of pigmentation onset were consistent with the progression of Stevens-Johnson syndrome.
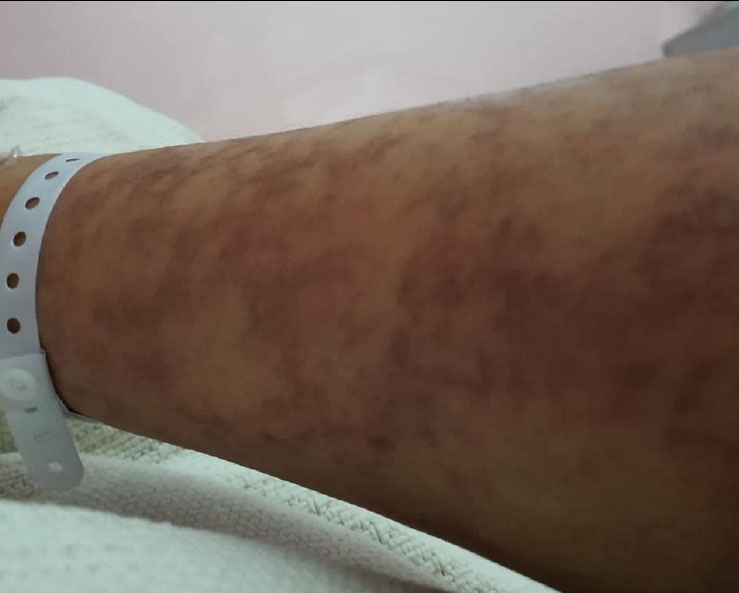
Patient’s arm exhibiting hyperpigmentation changes associated with Stevens-Johnson syndrome.
Fig. (3) shows a significant cracking and fissuring of the patient’s nails, which is a manifestation of Stevens-Johnson syndrome. These changes are attributed to the extensive damage to the epidermis and subsequent nail matrix involvement during the acute phase of Stevens-Johnson syndrome.
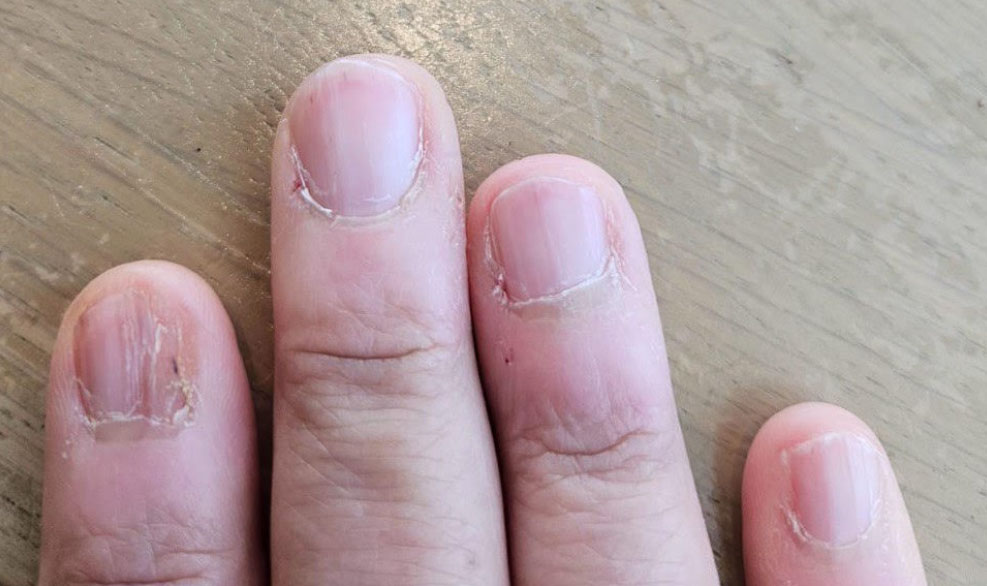
Patient’s nails showing significant cracking and fissuring, a manifestation of Stevens-Johnson syndrome.
2.1. Investigations
The laboratory findings indicated several concerning medical issues. Renal function showed a progressive decline, with the estimated glomerular filtration rate decreasing from 60 mL/min/1.73 m2 to 52 mL/min/1.73 m2 (Normal: ≥ 90 mL/min/1.73 m2) [4] while creatinine levels remained consistently elevated (Normal: 0.6-1.2 mg/dL), suggesting worsening kidney function [5]. Liver enzyme tests revealed that lactate dehydrogenase was elevated at 235 U/L (Normal: 140 - 280 U/L), whereas alanine aminotransferase and aspartate aminotransferase levels were within normal limits (Normal: ALT: 7-56 U/L; AST: 10-40 U/L) but showed slight variations [6].
Hematological findings indicated elevated mean corpuscular hemoglobin (Normal: 27-32 pg) and mean platelet volume (Normal: 7.5-11.5 fL), suggesting potential anemia and platelet activation [7]. Additionally, C-reactive protein levels were persistently elevated at 11.010 mg/L (Normal: <3 mg/L), indicating ongoing systemic inflammation [8]. Lastly, immunological markers revealed reduced complement levels, with C3 at 1.27 g/L (Normal: 0.9-1.8 g/L) and C4 at 0.32 g/L (Normal: 0.1-0.4 g/L), which was consistent with an autoimmune disorder [9]. These findings collectively highlighted the need for further evaluation and management of kidney health, inflammatory processes, and possible autoimmune conditions.
A biopsy of the minor salivary gland revealed lymphoid aggregates with more than 2 foci per 4 mm2, confirming the diagnosis of Sjögren’s syndrome.
Table 2 shows the detailed laboratory findings of the patient.
2.2. Management
Management of this patient required a multidisciplinary approach, focusing on symptomatic relief, preventing further organ damage, and addressing the complications of her comorbid conditions. Her treatment included hydroxychloroquine for managing the autoimmune response and pilocarpine to stimulate saliva production [10, 11]. Sugar-free gum and eye lubricants were given to alleviate the dryness of her mouth and eyes. Given her history of Stevens-Johnson syndrome, trachoma, and vitiligo, careful selection of medications was necessary to avoid triggering further complications. Regular monitoring of her kidney and liver function, as well as her overall systemic inflammation, was essential during the course of her overall management.
| Test Type | Parameter | Result | Reference Range | Interpretation | Laboratory Instrument Specifications |
|---|---|---|---|---|---|
| Renal Function | Estimated Glomerular Filtration Rate (eGFR) | 52 mL/min/1.73 m2 (declined from 60) | ≥90 mL/min/1.73 m2 | Progressive renal dysfunction | Automated Chemistry Analyzers: Roche Cobas® 6000 and 8000 Series Manufacturer: Roche Diagnostics Location: Indianapolis, Indiana & Siemens ADVIA Centaur XP Immunoassay System Manufacturer: Siemens Healthineers Location: Tarrytown, New York |
| Creatinine | Elevated | 0.6-1.2 mg/dL | Consistent with worsening kidney function | ||
| Liver Function | Lactate Dehydrogenase (LDH) | 235 U/L | 140-280 U/L | Elevated, indicating potential tissue damage or stress | |
| Alanine Aminotransferase (ALT) | Normal | 7-56 U/L | Within normal limits, with slight variations observed | ||
| Aspartate Aminotransferase (AST) | Normal | 10-40 U/L | Within normal limits, with slight variations observed | ||
| Hematology | Mean Corpuscular Hemoglobin (MCH) | Elevated | 27-32 pg | Suggestive of potential anemia or altered red blood cell function | Hematology Analyzers: Sysmex XN-2000 Manufacturer: Sysmex Corporation Location: Lincolnshire, Illinois Beckman Coulter® LH 750 Manufacturer: Beckman Coulter Location: Brea, California |
| Mean Platelet Volume (MPV) | Elevated | 7.5-11.5 fL | Indicates potential platelet activation or systemic inflammation | ||
| Inflammatory Markers | C-Reactive Protein (CRP) | 11.01 mg/L | <3 mg/L | Signifies ongoing systemic inflammation | Immunoassay Analyzers: Roche Cobas® 6000 and 8000 Series Manufacturer: Roche Diagnostics Location: Indianapolis, Indiana Siemens ADVIA Centaur XP Immunoassay System Manufacturer: Siemens Healthineers Location: Tarrytown, New York |
| Immunology | Complement C3 | 1.27 g/L | 0.9-1.8 g/L | Reduced, consistent with an autoimmune disorder | |
| Complement C4 | 0.32 g/L | 0.1-0.4 g/L | Reduced, consistent with an autoimmune disorder | ||
| Histopathology | Minor Salivary Gland Biopsy | Lymphoid aggregates (>2 foci/4 mm2) | N/A | Confirmed Sjögren’s syndrome diagnosis | Tissue Processors and Stainers: Leica Biosystems Tissue Processors Manufacturer: Leica Biosystems Location: Buffalo Grove, Illinois Sakura Tissue-Tek® Prisma® Stainer Manufacturer: Sakura Finetek USA, Inc. Location: Torrance, California |
3. DISCUSSION
Sjögren's syndrome is a chronic autoimmune disease characterized by lymphocytic infiltration of exocrine glands, primarily affecting the salivary and lacrimal glands, leading to the hallmark symptoms of xerostomia (dry mouth) and keratoconjunctivitis sicca (dry eyes). However, the impact of Sjögren's syndrome extends beyond these manifestations, involving multiple organ systems and resulting in significant morbidity. Sjögren's syndrome is a systemic disease with a broad range of extra-glandular manifestations. One of the most serious systemic complications is renal involvement, particularly in the form of tubulointerstitial nephritis. Tubulointerstitial nephritis is caused by lymphocytic infiltration of the kidneys, which can lead to significant renal impairment if not identified and treated early. Additionally, pulmonary involvement, including interstitial lung disease, is a significant concern. Interstitial lung disease can lead to progressive lung damage and fibrosis, contributing to considerable morbidity and mortality among patients with Sjögren's syndrome [12].
Neurological complications are also well-documented in Sjögren's syndrome, with both central and peripheral nervous system involvement. Peripheral neuropathy is the most common neurological manifestation, often presenting as sensory disturbances or neuropathic pain. Central nervous system involvement, although less frequent, can lead to more severe conditions, such as multiple sclerosis-like diseases, making it crucial for clinicians to be aware of these potential complications [13].
Oral complications are among the most prominent and challenging aspects of Sjögren's syndrome. The destruction of salivary glands leads to xerostomia, which is associated with a high risk of dental caries, oral infections, and difficulties in speaking and swallowing. According to a recent study, even with good oral hygiene practices, patients with Sjögren's syndrome often experience severe dental decay and tooth loss due to the persistent lack of saliva. The study emphasizes that root caries, which are less common in the general population, are particularly prevalent in Sjögren's syndrome patients due to the compromised salivary flow [14].
Patients with Sjögren's syndrome are at an increased risk of developing other autoimmune conditions, as well as serious complications, such as non-Hodgkin's lymphoma. The risk of non-Hodgkin's lymphoma, particularly the mucosa-associated lymphoid tissue subtype, is significantly higher in patients with Sjögren's syndrome compared to the general population. Persistent lymphadenopathy, particularly in the parotid glands, is often a precursor to lymphoma in these patients, highlighting the need for regular monitoring [15].
The present case illustrates the complexity of managing Sjögren’s syndrome, especially when it involves severe glandular dysfunction and systemic involvement. The oral manifestations, such as xerostomia, blocked salivary glands, and the near non-existence of salivary function, pose significant challenges, increasing the risk of dental caries, oral infections, and difficulty in swallowing. The presence of lymphoid aggregates in the biopsy confirms the autoimmune destruction of the salivary glands, a hallmark of Sjögren’s syndrome [16].
The patient’s history of Stevens-Johnson syndrome necessitates caution in prescribing medications, as this condition can be triggered by certain drugs. As practitioners are aware that Stevens-Johnson syndrome is a severe skin reaction that can occur due to various drugs used in daily practice, it is of utmost importance that careful selection of medications is done, especially keeping a keen eye on the patient during treatment. Sjögren’s disease complicates matters further as the autoimmune nature of the disease can precipitate and exaggerate pre-existing conditions or any other immune response like Stevens-Johnson syndrome.
Another complication worth noting in the patient was a history of stroke. Patients with Sjögren’s have long been known to display characteristics of increased coagulation and vascular inflammation, which can lead to atherosclerosis and subsequent stroke [17]. Concurrently, it is important to highlight that such a patient taking oral contraceptives may experience adverse effects on health, as hormonal influences and endothelial dysfunction can cause cerebral venous thrombosis [13]. Therefore, risk assessment and weighing the advantages of treatment must be meticulously considered before commencing such a regimen in predisposed patients, including those with hypertension and prediabetes.
One of the pathognomonic signs of Sjögren’s syndrome is dryness of the eyes, which can have deleterious effects on the conjunctiva and provide opportunistic infections a chance to develop. The patient in this study was unfortunately exposed to such an infection and subsequently developed trachoma. While trachoma is infectious, some studies suggest that individuals with autoimmune disorders could be at a higher risk for various infections, including trachoma, caused by the bacterium Chlamydia trachomatis. It is characterized by inflammation of the conjunctiva and cornea, leading to scarring and potentially blindness if untreated [14, 18].
The presence of Sjögren’s syndrome often signals a higher likelihood of other autoimmune disorders, necessitating comprehensive monitoring and management strategies for affected patients, which can add another layer of complexity. In this case, the patient also presented with another autoimmune disease, vitiligo. Although both diseases are distinct in nature, affecting different systems of the body, studies discussing the pathophysiology of autoimmune diseases have indicated that inflammatory processes and immune dysregulation are common features linking Sjögren's syndrome and vitiligo [14]. Vitiligo primarily affects skin melanocytes, wherein autoantibodies destroy these cells, causing loss of pigmentation and leading to scattered white patches over the skin, resulting in cosmetic challenges. The presence of Sjögren’s definitely predisposes individuals to develop other autoimmune conditions, necessitating thorough investigation to rule out such diseases that can complicate overall management [13].
The elevated C-reactive protein levels and fluctuations in liver enzymes suggest ongoing systemic inflammation, which could exacerbate Sjögren’s symptoms and contribute to further organ involvement. The decline in renal function, as indicated by the drop in estimated glomerular filtration rate and elevated creatinine levels, highlights the importance of regular monitoring and early intervention to prevent further deterioration. Furthermore, the management of Sjögren's syndrome is complicated by the presence of comorbid conditions, such as hypertension, prediabetes, and a history of severe drug reactions like Stevens-Johnson syndrome. These comorbidities require careful management to avoid exacerbating the primary disease or triggering additional complications [2, 11].
The multifaceted challenges of managing Sjögren's syndrome, particularly in patients with severe glandular dysfunction and multiple comorbidities, highlight the necessity for individualized care and multidisciplinary strategies. Current treatment approaches, such as immunomodulatory drugs like hydroxychloroquine and local interventions like saliva stimulants, offer symptomatic relief but often fail to address the full spectrum of complications associated with the disease. The development of more targeted therapies remains a critical area of research.
Recent studies have explored adjunctive treatments that may offer additional benefits in managing Sjögren's syndrome. Ozone therapy has demonstrated promise in modulating inflammatory responses and enhancing periodontal healing, as seen in comparisons between ozonized gels and chlorhexidine for periodontal treatment [19]. This raises the possibility of its application in managing oral complications associated with Sjögren’s syndrome [20].
Photobiomodulation has also been investigated for its potential benefits. A study demonstrated its efficacy in reducing injection pain in pediatric patients, suggesting its anti-inflammatory and analgesic potential, which could be explored further for managing xerostomia and oral discomfort in Sjögren's syndrome patients [21, 22]. Paraprobiotics, non-viable microbial cells that exert beneficial effects on host health, have shown promise in managing periodontitis, as evidenced by studies on pregnant women [23]. This novel approach could be particularly beneficial for modulating the oral microbiome and reducing systemic inflammation in Sjögren’s syndrome. Additionally, emerging biologic agents targeting cytokines, signal pathways, and immune responses have shown efficacy in treating primary Sjögren's syndrome [24].
These findings underscore the importance of continued research into adjunctive therapies that may offer relief to patients with Sjögren's syndrome. Future studies should focus on further evaluating the efficacy and safety of these treatments, both individually and in combination, to establish their roles in comprehensive patient care. Investigating the mechanisms through which these therapies influence immune modulation and glandular function is crucial for optimizing outcomes.
4. LIMITATIONS
While this case report provides valuable insights into the complex interplay of Sjögren’s syndrome and its co-morbidities, certain limitations should be acknowledged. Being a single case study, the findings are inherently specific to the patient and may not fully represent the broader spectrum of individuals with this condition. The retrospective nature of the report relies on the patient’s medical history, which could introduce incomplete or selective data. Additionally, while the discussion highlights potential adjunctive therapies, such as ozone therapy and photobiomodulation, these were not explored in the current case, leaving room for future investigations. The focus on oral health and systemic implications, though significant, could benefit from a more detailed analysis of long-term outcomes and alternative management strategies.
CONCLUSION
This case highlights the multifaceted challenges of managing Sjögren’s syndrome in a patient with severe glandular dysfunction and a complex medical history. The combination of severe xerostomia, systemic involvement, and multiple comorbidities necessitates a nuanced, individualized approach to care. Early detection, regular monitoring, and a multidisciplinary treatment strategy are essential to managing the patient’s condition effectively, reducing the risk of further complications, and improving her overall quality of life. In conclusion, this case highlights the significance of thorough monitoring and management in patients with Sjögren’s disease, especially when there is systemic involvement and several comorbidities. Timely identification and customized treatment strategies are essential for addressing the complex difficulties presented by such illnesses, subsequently improving patient results. This case underscores the importance of a comprehensive, multidisciplinary approach to managing Sjögren’s syndrome, particularly in patients with complex medical histories. Regular monitoring of organ function, careful medication management, and targeted therapies to address both the autoimmune symptoms and the complications of her comorbid conditions are crucial to improving her quality of life and preventing further complications.
AUTHOR’S CONTRIBUTION
A.M., H.A.A., and M.S.A.: Study conception and design; A.M. and A.B.A.: Data collection; A.L.A. and S.F.A.: Analysis and interpretation of results; R.A.A. and R.I.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| eGFR | = Estimated Glomerular Filtration Rate |
| LDH | = Lactate Dehydrogenase |
| ALT | = Alanine Aminotransferase |
| AST | = Aspartate Aminotransferase |
| MCH | = Mean Corpuscular Hemoglobin |
| MPV | = Mean Platelet Volume |
| CRP | = C-Reactive Protein |
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article are available within the article.
ACKNOWLEDGEMENTS
Declared none.


