All published articles of this journal are available on ScienceDirect.
Successful Treatment of Cheilitis Glandularis Accompanied by Pseudomonas aeruginosa Infection: A Case Report
Abstract
Background
Cheilitis glandularis (CG) is a rare inflammatory disorder affecting the minor salivary glands. Nonetheless, the ideal management of CG remains elusive. Hence, this study aimed to broaden the understanding of the etiopathogenesis of CG and recommend bacterial culture and drug sensitivity tests for CG patients with suspected infection.
Case Presentation
A 78-year-old female came to our hospital complaining of painful protrusion in her right lower labial mucosa for three weeks. Clinical examination revealed local enlargement with pyorrhea on the right lower labial mucosa. Histopathological examination indicated the features of CG. Bacterial culture and drug sensitivity test demonstrated Pseudomonas aeruginosa (P. aeruginosa) infection with several susceptible antibiotics. The patient was treated with surgical excision of the right lower labial mucosa combined with levofloxacin daily for two weeks. At the 4-week follow-up after excision, only a small amount of scar remained in the excision area. The patient had no symptoms in the oral mucosa.
Conclusion
CG may be accompanied by P. aeruginosa infection. Bacterial culture and drug sensitivity tests may contribute to identifying potential pathogens in CG patients and improving the efficacy of the treatment.
1. INTRODUCTION
Cheilitis glandularis (CG) is a rare inflammatory disorder that is more common in males in their 40 years of age [1, 2]. The clinical manifestation of CG is characterized by the dilation of the orifices of the minor salivary glands [3, 4]. The etiology of CG remains obscure. Multifactorial factors, including long-term smoking, diabetes mellitus, drug-induced xerostomia, actinic damage, immunosuppression, poor oral hygiene, local trauma, and even heredity may contribute to the occurrence, development, and aggravation of the condition [5]. CG tends to be chronic, and it can be classified as simplex, superficial suppurative, and deep suppurative subtypes at different stages of CG progression [4, 6].
Treatment strategies for CG vary in accordance with the severity of symptoms. Apart from conventional treatments, which consist of surgical procedures and the administration of corticosteroids, the prescription of antibiotics is also frequently necessary [7, 8]. There are various classifications of antibiotics for different types of bacteria. Whether and how to choose antibiotic treatment are essential events in the process of clinical decision-making for the treatment of CG.
In this article, we have reported a case of CG accompanied by Pseudomonas aeruginosa (P. aeruginosa) infection, which has been successfully treated with surgical procedures and levofloxacin, providing an insight into the etiopathogenesis of CG.
2. CASE PRESENTATION
A 78-year-old female visited our department at Shanghai Ninth People’s Hospital complaining of a mild painful protrusion in her right lower labial mucosa for three weeks. She had taken azithromycin enteric-coated capsules 250 mg twice daily and applied 0.03% tacrolimus ointment once daily for two weeks. Unfortunately, no improvement was observed, and impairment of normal eating persisted. The patient denied symptoms of xerostomia or xerophthalmia, and she also denied traumatic factors on the lower labial mucosa. Systemically, the patient was diagnosed with hypertension 20 years ago, but did not take any antihypertensive drugs despite her unstable blood pressure control. Actinic damage, family history, smoking, and alcohol consumption were all negative.
Clinical examination demonstrated local enlargement in the right lower labial mucosa. On the center of the enlarged lesion, pyorrhea was observed (Fig. 1A). The lesion was 3 mm in diameter, and the texture was homogeneously soft. Periodontal examination revealed gingival recession, calculus formation, and clinical attachment loss of 7 mm, 7 mm, 4 mm, and 5mm in teeth #41, #42, #43, and #44, respectively. Grade I mobility was detected in tooth #44, and grade II mobility was detected in teeth #41 and #42. The percussion of the teeth #41, #42, #43, and #44 and the palpation of the buccal soft tissues were negative. No gingival fistula in the periapical area was observed. No other abnormal lesions were found on oral mucosa or maxillofacial regions. Laboratory tests revealed blood routine, coagulation function and C-reactive protein indices to be within the normal range. To detect possible pathogens and confirm the diagnosis of the oral lesions, bacterial culture, drug sensitivity test, and biopsy were performed on the right lower labial mucosa. Histopathological findings demonstrated enlarged salivary ducts, epithelial hyperplasia of ducts, inflammatory lymphocytes, and plasma cell infiltration around the ducts (Fig. 2A-B). Bacterial culture indicated P. aeruginosa infection. The result of anti-bacterial drug sensitivity test revealed several antibiotics, including gentamicin, ciprofloxacin, levofloxacin, piperacillin/tazobactam, cefotaxime, cefepime, imipenem, amikacin, tobramycin, cefoperazone/sulbactam, meropenem, and Azuron as susceptible (Table 1). Based on the clinical findings, histopathological features, and the results of bacterial culture, a final diagnosis of CG accompanied by P. aeruginosa infection was made.
The patient was subsequently treated with surgical excision of the right lower labial mucosa combined with the administration of levofloxacin 250 mg three times daily for two weeks. At two weeks after the excision, the wound healed, and pyorrhea disappeared without any adverse event (Fig. 1B). At the 4-week follow-up after excision, only a small amount of scar remained in the excision area (Fig. 1C). The patient had no symptoms in the oral mucosa and was pleased with the effect of the treatment. The patient was suggested to undergo treatment for periodontal diseases.
3. DISCUSSION
This report has presented a case of CG accompanied by P. aeruginosa infection. To the best of our knowledge, this is the first time that P. aeruginosa infection has been identified in patients with CG. Our case has suggested that it is necessary to carry out bacterial culture and drug sensitivity tests for CG patients when infection is suspected.
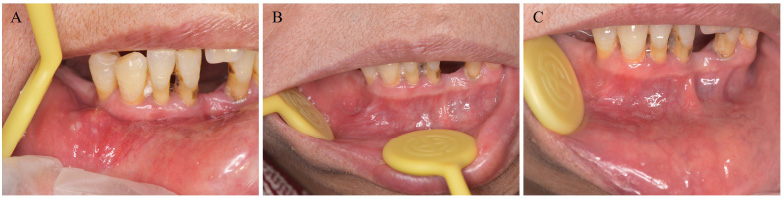
Clinical manifestations of oral mucosa before and after treatment. (A) Local enlargement and pyorrhea of right lower labial mucosa before treatment; (B) Two weeks after excision of enlargement of right lower labial mucosa; (C) Four weeks after excision of enlargement of right lower labial mucosa.
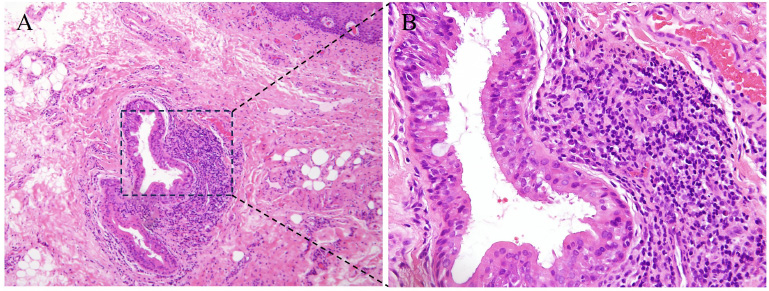
Histopathological features of the right lower labial mucosa. (A) hematoxylin and eosin, 100×; (B) enlargement of dashed box in panel A, 200×.
| Drug | Minimum Inhibitory Concentration | Degree of Sensitivity |
|---|---|---|
| Gentamicin | ≤ 1 | Susceptible |
| Ciprofloxacin | 0.5 | Susceptible |
| Levofloxacin | 0.5 | Susceptible |
| Piperacillin/tazobactam | ≤ 4 | Susceptible |
| Cefotaxime | ≤ 1 | Susceptible |
| Cefepime | ≤ 1 | Susceptible |
| Imipenem | ≤ 1 | Susceptible |
| Amikacin | ≤ 2 | Susceptible |
| Tobramycin | ≤ 1 | Susceptible |
| Cefoperazone/sulbactam | Not applicable | Susceptible |
| Meropenem | Not applicable | Susceptible |
| Azuron | Not applicable | Susceptible |
The etiopathogenesis of CG has been found to be multifactorial; however, the specific etiopathogenesis is still unclear. Actinic damage and smoking have been reported in the literature as possible causative factors [9]. However, in a recent study, only one out of fourteen patients with CG presented with actinic cheilitis, indicating that actinic damage may not be a significant predisposing factor for CG. Long-term smoking-induced xerostomia might also participate in the initial pathogenesis of CG [5]. In this case, the patient denied the history of actinic damage, smoking, and the symptoms of xerostomia. In addition, poor oral hygiene, which acts as a local irritant, might have further contributed to the development and aggravation of CG [5], which explains why CG developed in our patient. Based on this, periodontal management was advised to our patient. The exact etiopathogenesis of CG needs to be explored in further fundamental and clinical studies.
Currently, the ideal management of CG remains elusive without a definitive consensus. Systemic antibiotics, along with sunscreen, topical and intralesional steroids, topical calcineurin inhibitors, and surgical therapy, have been proposed for its treatment [2, 4, 5, 9]. The treatment efficacy of systemic antibiotic usage has been reported as controversial. In a case of CG patient who received systemic antibiotic clarithromycin for 3 months and subsequently, doxycycline for 3 months, no improvement was observed [9], while minocycline in combination with topical tacrolimus ointment 0.1% was employed in another case, which led to the recovery of the lip [7]. Based on bacterial culture, researchers identified Staphylococcus aureus infection in a case of CG. Clindamycin was administered intravenously, and surgical treatment was performed, which resulted in a good curative effect [8]. Referring to the above case, we have carried out bacterial culture and drug sensitivity tests and achieved good clinical effects through surgical treatment combined with the use of an oral antibiotic.
In this case, P. aeruginosa infection was identified on the labial mucosa. As an aerobic Gram-negative bacteria and opportunistic pathogen, P. aeruginosa has various strains and is frequently resistant to several antibiotics through multiple chromosomal determinants as well as the complex regulatory pathways involved in intrinsic and adaptive resistance [10, 11]. P. aeruginosa infection is closely related to oral diseases. The prevalence of P. aeruginosa in epithelial cells was correlated with the state of periodontal disease [12]. A direct relationship between the use of removable orthodontic appliances and the increase of P. aeruginosa infection has also been established [13]. In our case, for the first time, P. aeruginosa infection accompanied by CG has been reported, indicating the significance of bacteria culture whenever the infection is suspected.
There exist some limitations related to this study. First, due to the short follow-up time of this case, it was difficult to evaluate the long-term recurrence of CG and P. aeruginosa infection. Second, considering the nature of the case report, further studies are needed to clarify the relationship between CG and P. aeruginosa infection.
CONCLUSION
In conclusion, CG may be accompanied by P. aeruginosa infection. Moreover, bacterial culture and drug sensitivity tests may contribute to identifying potential pathogens in CG patients and improving the efficacy of treatment.
AUTHORS' CONTRIBUTION
H.S.: Interpreted the patient’s data and wrote the original draft; K.S.: Collected clinical images, undertook excision, and performed the histological examination; Y.W.: was a major contributor to reviewing and editing the manuscript. All authors read and approved the final manuscript.
LIST OF ABBREVIATIONS
| CG | = Cheilitis Glandularis |
| P. aeruginosa | = Pseudomonas aeruginosa |
ETHICAL STATEMENT
This study was approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, China (SH9H-2024-T317-1).
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation.
CONSENT FOR PUBLICATION
The patient gave written consent for her personal and clinical details, along with any identifying images to be published in this study.
AVAILABILITY OF DATA AND MATERIALS
The data used to support the findings of this study are included in the article.
FUNDING
This work was funded by the Health Discipline Construction Project in Pudong New Area, No. PWYts2021-20.
ACKNOWLEDGEMENTS
The authors are grateful to the patient for follow-up, which contributed to this study. They would also like to thank Prof. Lizhen Wang and Dr. Rong-Hui Xia from the Department of Oral Pathology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine for providing a pathological diagnosis in this case.


