All published articles of this journal are available on ScienceDirect.
Properties of Citrullus Colocynthis as a Pulpotomy Medicament: An Animal Study
Abstract
Introduction
As the carcinogenic properties of formocresol spark global concern, the exploration of safer alternatives becomes paramount in pediatric dentistry. This study assesses the efficacy of Citrullus colocynthis extract, recognized for its potent anti-inflammatory and antimicrobial qualities, as a potential substitute in pulpotomy treatments.
Methods and Materials
Employing a controlled experimental design, eight New Zealand white rabbits underwent pulpotomy using either the traditional formocresol or the novel Citrullus colocynthis extract. Following the treatments, comprehensive histological assessments were performed to evaluate inflammatory responses, tissue necrosis, and morphological changes in pulp cells. Data analysis was conducted with SPSS, utilizing Chi-square and Mann Whitney tests to ascertain statistical significance.
Results
Statistically significant disparities were evident in the levels of inflammation, infiltration of inflammatory cells, and morphological changes of pulp tissue cells, with the Citrullus colocynthis extract group showing more pronounced adverse effects. In contrast, rates of necrosis did not differ significantly between the two groups.
Conclusion
Despite its notable anti-inflammatory properties, Citrullus colocynthis extract induced significant adverse cellular reactions in pulpotomy applications. While promising as a therapeutic agent, its potential cellular toxicity suggests a cautious approach to clinical usage in dental treatments, warranting further investigation and optimization for safety.
1. INTRODUCTION
Tooth decay is a pervasive chronic infectious disease that is both treatable and preventable [1]. Treatment of primary tooth caries varies depending on its extent and the presented signs and symptoms, necessitating diverse dental interventions. Preserving primary teeth in a healthy functional state is crucial for maintaining not only arch length but also a child's aesthetic appearance, mastication, speech, and oral-facial development. Therefore, the principal aim of pediatric dentistry is to uphold arch space by retaining natural deciduous teeth until the eruption of their permanent successors [2, 3].
Long-standing research by dental scholars has focused on treating pulp diseases and exposures in primary and young permanent teeth, whether due to decay or trauma. The quest for the ideal medicament to cover the exposed pulp post-treatment has long been a matter of debate [4]. Pulpotomy, the procedure that entails the coronal pulp's removal, aims to preserve the vitality of the tooth and promote pulp healing. The overarching goal of pulpal therapy in children is to ensure the health and function of deciduous teeth until permanent ones emerge [5, 6].
The history of pulpotomy dates back to 1904, when Buckley introduced formocresol, which was later popularized by Sweet in 1930 as a pulpotomy technique for primary teeth [7]. However, concerns have risen since the International Agency for Research on Cancer classified formaldehyde, a component of formocresol, as a carcinogen linked to leukemia and nasopharyngeal carcinoma in 2004 [8]. Despite the clinical efficacy of formocresol, its potential for causing sensitization, cytotoxicity, mutagenicity, carcinogenicity, and harmful fetal effects has driven researchers to seek safer alternatives. In this quest, medicinal plant extracts have garnered attention due to their safety, natural composition, and affordability [9-11].
The use of medicinal plants in healthcare, disease prevention, and treatment has significantly increased globally over the years. These plants are not only dietary staples and primary materials but are also reservoirs of diverse chemicals and natural molecules that have catalyzed the development of various medications [12]. The desert plant Citrullus colocynthis, known for its myriad bioactive compounds—fatty acids, glycosides, flavonoids, alkaloids, and essential oils—has been extensively studied for its antibacterial, antioxidant, and anti-inflammatory properties, leading to its use in addressing diabetes, inflammation, parasitic infections, and cancer [13].
Citrullus colocynthis, commonly known for its extensive medicinal applications, has demonstrated significant antimicrobial properties. Research shows that extracts from the fruit and seeds of C. colocynthis are effective against both gram positive and gram negative bacteria, including Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Enterococcus faecalis [14, 15]. These findings underscore its potential as an alternative to traditional antimicrobials in treating bacterial infections.
In traditional medicine, C. colocynthis has been valued for its efficacy in treating a range of disorders, from digestive issues to chronic diseases. It is notably used for indigestion, gastroenteritis, diabetes, and liver problems, as well as for relieving pain due to its analgesic properties [16, 17]. Additionally, it is applied in the management of respiratory and gastrointestinal ailments like colds, diarrhea, and bronchitis and is also used for reducing fever [18].
The multifaceted efficacy of Citrullus colocynthis not only highlights its potential as a comprehensive therapeutic agent but also underscores the importance of further research to unlock its full potential in clinical settings, particularly in pulpotomy and other dental applications. The promising results suggest a pathway for its integration into modern medical practices, albeit with careful consideration of its dosage and toxicity to optimize safety and effectiveness. References to the efficacy of Citrullus colocynthis and its extract for toothache relief are noted in the traditional medicinal teachings of Iran and Pakistan. Considering the extensive research on pulpotomy-related properties of various traditionally used plants and the recognized antimicrobial and anti-inflammatory qualities of Citrullus colocynthis, this study aims to investigate the effectiveness of its ethanol extract in animal models for pulpotomy treatment [16, 19, 20].
2. METHODS AND MATERIALS
This study was conducted adhering to ethical guidelines for laboratory research, with a commitment to environmental preservation and humane treatment of animals. Ethical approval was obtained from the Baqiyatallah University of Medical Sciences' ethical committee [Ethics Code: IR.BMSU.BAQ.REC.1398.057] prior to the initiation of research activities.
2.1. Plant Extraction Process
The Citrullus colocynthis plants used in our study were obtained from the Medicinal Plants and Drugs Research Institute of Shahid Beheshti University. Further information about the institute can be found on their official website: https://mpdri.sbu.ac.ir/.The process began with the drying of Citrullus colocynthis fruits under shade. To prepare the ethanolic extract, 400 grams of these dried fruits were ground and macerated in a 70:30 ethanol-water solution, totaling 1.2 liters, for three days. The solution was stirred twice for optimal extraction. Post-maceration, the solution was filtered three times. After the ethanol was evaporated using a rotary evaporator, the remaining water was separated through lyophilization, and the freeze-dried extract was obtained [21, 22].
2.2. Animal Intervention
The rabbits used in our study were purchased by the Animal Center of Baqiyatallah University of Medical Sciences from the Pasteur Institute of Iran. Below are the relevant details:
Supplier Name: Pasteur Institute of Iran, Address: No. 69, Pasteur Ave, Tehran, Iran
Eight male New Zealand White rabbits, aged 14.4 ± 2.1 weeks and weighing 2 kg ± 200 g, were used in this study. The rabbits were housed in individual steel cages within a sterile room at the Animal Center of Baqiyatallah University of Medical Sciences. The environmental conditions were carefully controlled, maintaining a temperature of 24°C ± 2°C, relative humidity of 60%, light intensity of 350–400 lux, noise levels below 85 dB, and a 12-hour light/dark cycle. The animals were acclimatized to these conditions for three days before cavity preparation and for eight days before euthanasia.
The rabbits were provided with unlimited access to dried food pellets (Witte Molen, Netherlands) and fresh water. All procedures were conducted in accordance with the ethical approval obtained for the study.
For anesthesia, a combination of ketamine (35 mg/kg) and xylazine (5 mg/kg) was administered intramuscularly. Following anesthesia, the anterior maxillary and mandibular regions were disinfected with a 2% chlorhexidine solution. Using a sterile high-speed handpiece and a round diamond bur, access cavities were created on the buccal surfaces of the central incisors [23, 24]. Pulp tissue removal was meticulously performed using a sterile spoon excavator. Hemostasis was achieved with sterile cotton balls moistened with saline (Fig. 1). A simple randomisation method was used to allocate teeth to treatment groups. A random number generator was employed to assign one tooth per rabbit to the formocresol group, while the remaining teeth were assigned to the Citrullus colocynthis extract group. This ensured the unbiased allocation of treatments across the samples. Through this process, the teeth were chosen to receive either formocresol or Citrullus colocynthis extract, applied via cotton balls for five minutes. Depending on the rabbits' cooperation and the effective duration of anesthesia, 2-3 teeth per rabbit were treated.
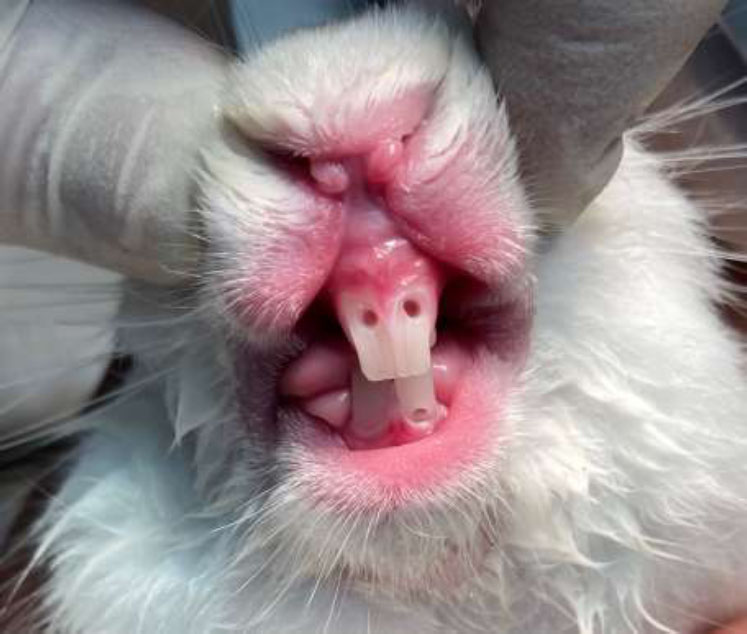
Preparation of access cavities on central incisors and hemostasis.
After the application of either formocresol or the Citrullus colocynthis extract, a zinc oxide mixture was applied to the cavities. For teeth treated with formocresol, the mixture consisted of zinc oxide and eugenol, whereas for those treated with the Citrullus colocynthis extract, a mixture of zinc oxide and the extract was used. Following this, the access cavities were sealed using a self-cured restorative glass ionomer (Fig. 2).
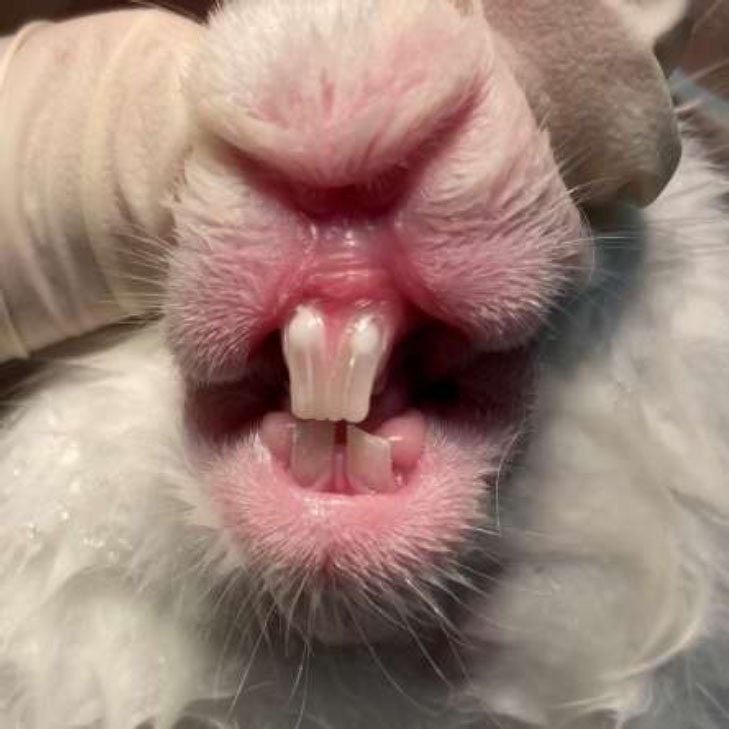
Restoring access cavities with glass ionomer.
Eight days following the treatment, the rabbits were euthanized using a triple dose of the anesthetic. The absence of any signs of vitality was confirmed, ensuring a humane endpoint before the sampling of the upper and lower jaws based on the teeth that received treatment [24].
2.3. Histological Examination
After the teeth were fixed in a 10% formaldehyde solution, they were sent to the laboratory. The process for preparing the samples for microscopic examination included decalcification in a 20% formic acid solution buffered with sodium citrate for ten days, longitudinal sectioning, dehydration, and embedding in paraffin. Following these steps, the samples were stained using the hematoxylin and eosin (H&E) method. This series of steps culminated in the preparation of slides for microscopic analysis. The histological findings are illustrated in Figs. (3-5).
Upon preparation, the slides underwent examination by a histopathologist under a microscope. The evaluation focused on:
2.3.1. Inflammation
Characterized by the dilation of blood vessels and an increase in interstitial fluid.
2.3.3. Infiltration of Inflammatory Cells
Primarily measured by the presence of macrophages within the tissue.
2.3.4. Morphological Change of Cells
Observed as alterations in the appearance and structure of cells present in the pulp tissue.
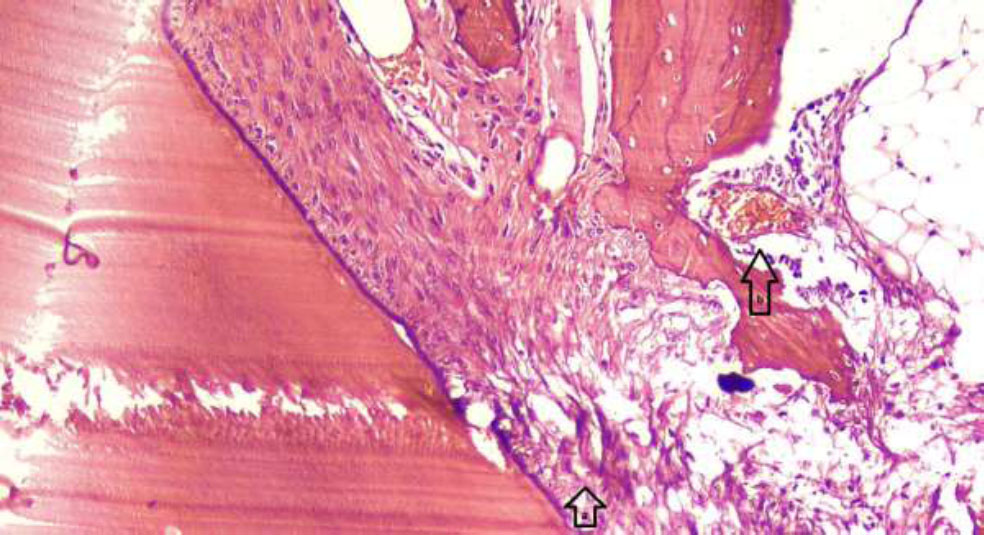
Histological image of a rabbit tooth treated with Citrullus colocynthis extract, showing localized morphological changes in pulp tissue and mild inflammation. (a) Arrows indicating localized morphological changes of pulp cells, with evidence of cell distortion and altered cell arrangement. (b) Dilation of blood vessels in response to inflammation, as marked by arrows, indicating increased vascularity in the affected area. The changes observed suggest a response to the treatment, with possible localized inflammation. Hematoxylin and eosin staining is shown with 100x magnification.
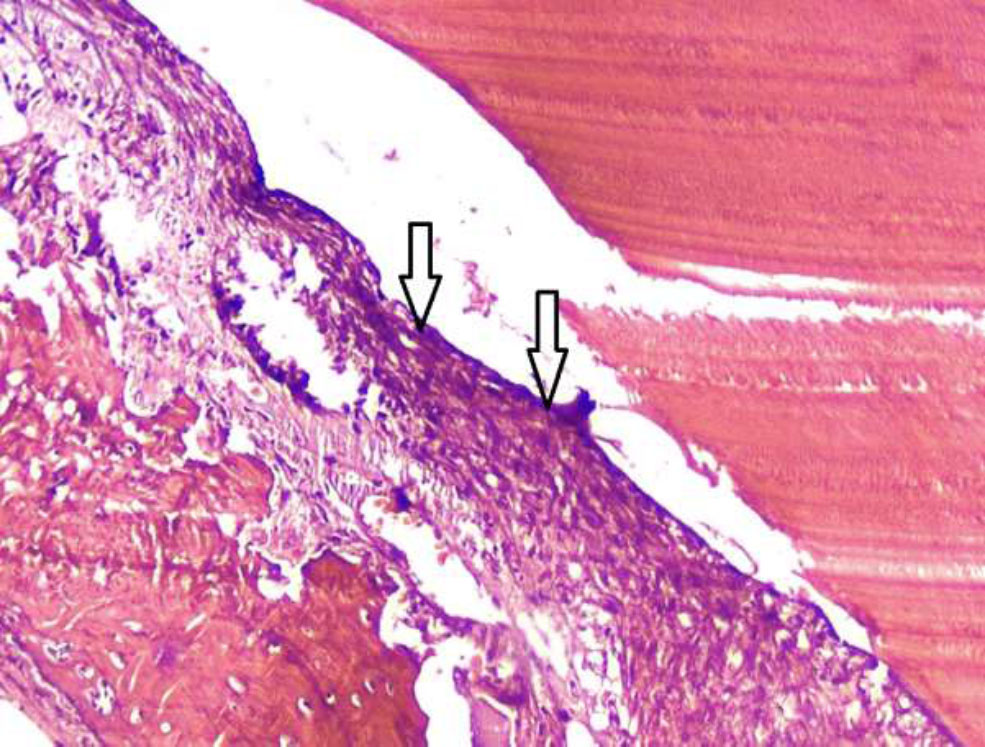
Histological image of a rabbit tooth treated with Citrullus colocynthis extract, showing the onset of localized necrosis. Arrows indicating the initial area of necrosis, where the pulp tissue begins to lose its normal structure. The necrotic region is still localized but has the potential to spread due to the extent of tissue damage. The affected area exhibits signs of cell death, with the absence of viable cells in the region. Hematoxylin and eosin staining is shown with 100x magnification.
The histopathologist conducted a qualitative assessment, categorizing the presence of inflammation and the infiltration of inflammatory cells as negative, low, moderate, or high, and assigned a rating from 0 to +3 based on the intensity and spread of the inflammation. Similarly, the assessment of necrosis and morphological changes in tissue was reported as negative, low and limited, or high and widespread, rated from 0 to +2.
It is important to note that not all pathological symptoms (e.g., inflammation, necrosis, morphological changes) may be visible in a single section of the tooth, as these changes can be focal and may occur at varying distances from the site of treatment. Therefore, the pathologist thoroughly examined all sections of each treated tooth to ensure comprehensive evaluation. Each section was scored individually, with the cumulative findings used to assess the overall response to treatment. This approach ensures that localized changes, which may not be present in every section, are adequately captured and considered in the final analysis.
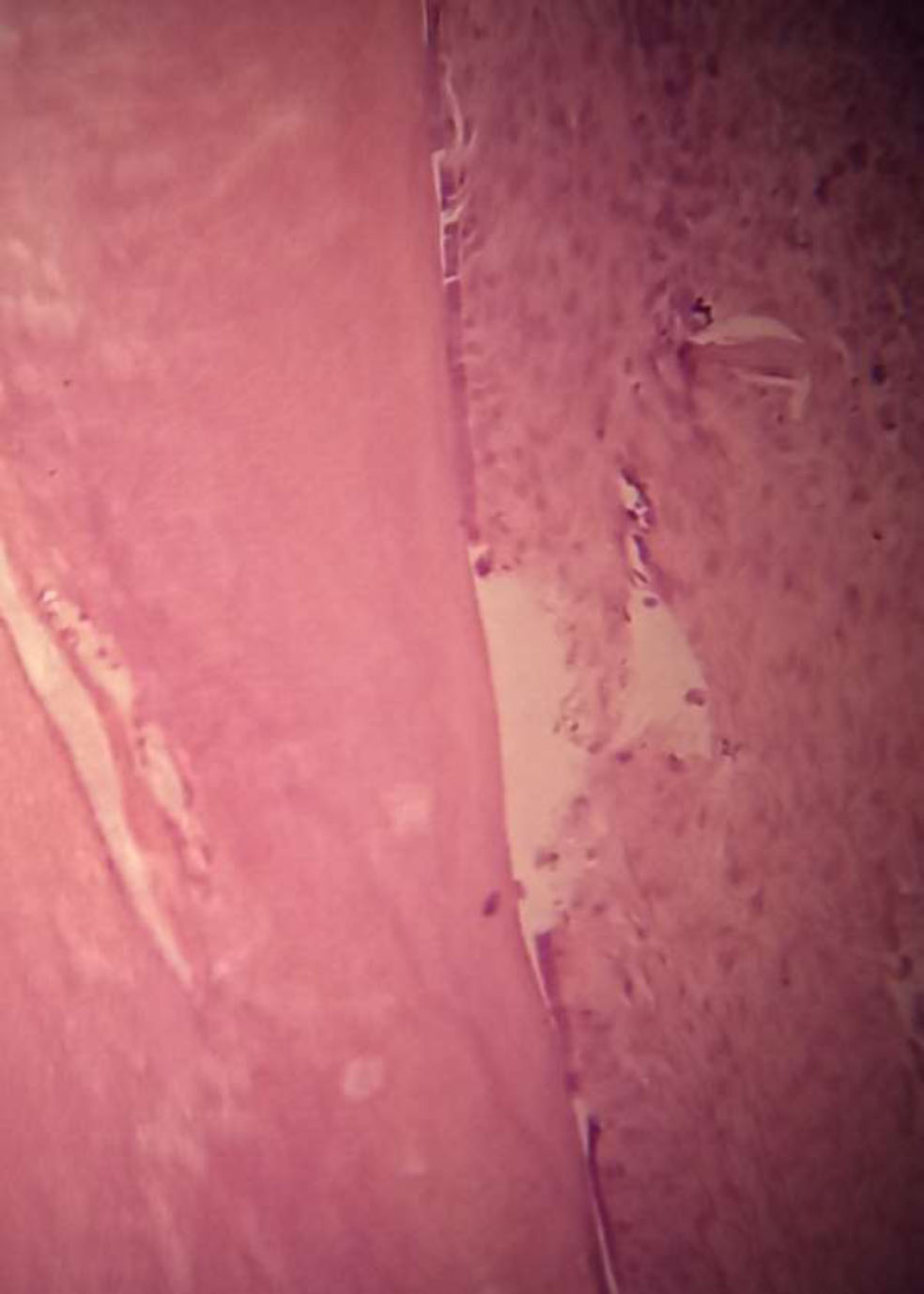
Histological image of a rabbit tooth treated with formocresol, showing no signs of inflammation or other adverse effects in this section. The pulp tissue appears healthy, with normal cellular architecture and no observable changes in morphology or vascular dilation. Hematoxylin and eosin staining is shown with 40x magnification.
3. RESULTS
3.1. Inflammation
Only 2 of the 7 samples treated with formocresol (28.6%) showed mild inflammation, while the other 5 samples (71.4%) exhibited no inflammation. In contrast, among the 13 samples treated with Citrullus colocynthis extract, 4 had no inflammation, 2 displayed mild inflammation, 4 had moderate inflammation, and 3 showed severe inflammation under microscopic examination (Table 1).
| - | Inflammation | - | Chi2 | |||||
|---|---|---|---|---|---|---|---|---|
| Negative | 1+ | 2+ | 3+ | Mann-Whitney | ||||
| Medicament | F. | Count | 5 | 2 | 0 | 0 | 0.032 | 0.029 |
| Percent | 71.4% | 28.6% | 0.0% | 0.0% | ||||
| C.c. | Count | 4 | 2 | 4 | 3 | |||
| Percent | 30.75% | 15.4% | 30.75% | 23.1% | ||||
| - | Necrosis | - | Chi2 | ||||
|---|---|---|---|---|---|---|---|
| Negative | 1+ | 2+ | Mann-Whitney | ||||
| Medicament | F. | Count | 5 | 2 | 0 | 0.101 | 0.09 |
| Percent | 71.4% | 28.6% | 0.0% | ||||
| C.c. | Count | 5 | 4 | 4 | |||
| Percent | 38.5% | 30.75% | 30.75% | ||||
3.2. Necrosis
Of the 7 cases treated with formocresol, 2 (28.6%) showed local necrosis, while no necrosis was observed in the remaining cases. In the samples treated with Citrullus colocynthis extract, out of 13, there were 4 instances of local necrosis, 4 of diffuse necrosis, and 5 with no tissue necrosis reported (Table 2).
3.3. Infiltration of Inflammatory Cells
This study focused mainly on the infiltration of macrophages. Only 1 of the 7 cases treated with formocresol (14.3%) showed mild infiltration, with the remaining 6 being negative. Among the cases treated with Citrullus colocynthis extract, out of 13, 5 were negative, 5 showed mild infiltration, 2 displayed moderate infiltration, and 1 case showed widespread infiltration of inflammatory cells (Table 3).
3.4. Morphological Change of Pulp Tissue
No morphological changes in pulp tissue cells were observed in any of the samples treated with formocresol. In comparison, among the 13 cases treated with Citrullus colocynthis extract, 3 showed severe morphological changes, 5 had mild changes, and 5 showed no changes (Table 4).
| - | Inflammatory Cell Infiltration | - | Chi2 | |||||
|---|---|---|---|---|---|---|---|---|
| Neg. | 1+ | 2+ | 3+ | Mann-Whitney | ||||
| Medicament | F. | Count | 6 | 1 | 0 | 0 | 0.043 | 0.05 |
| Percent | 85.7% | 14.3% | 0.0% | 0.0% | ||||
| C.c. | Count | 5 | 5 | 2 | 1 | |||
| Percent | 38.5% | 38.5% | 15.4% | 7.6% | ||||
| - | Morphologic Change | - | Chi2 | ||||
|---|---|---|---|---|---|---|---|
| Neg. | 1+ | 2+ | Mann-Whitney | ||||
| Medicament | F. | Count | 7 | 0 | 0 | 0.011 | 0.017 |
| Percent | 100.0% | 0.0% | 0.0% | ||||
| C.c. | Count | 5 | 5 | 3 | |||
| Percent | 38.5% | 38.5% | 23% | ||||
Based on the available findings and statistical tests, there were statistically significant differences between the two groups treated with Citrullus colocynthis extract and formocresol in terms of inflammation, infiltration of inflammatory cells, and morphological changes of pulp cells. However, no statistically significant difference was observed in the occurrence of necrosis between the two groups (Fig. 6).
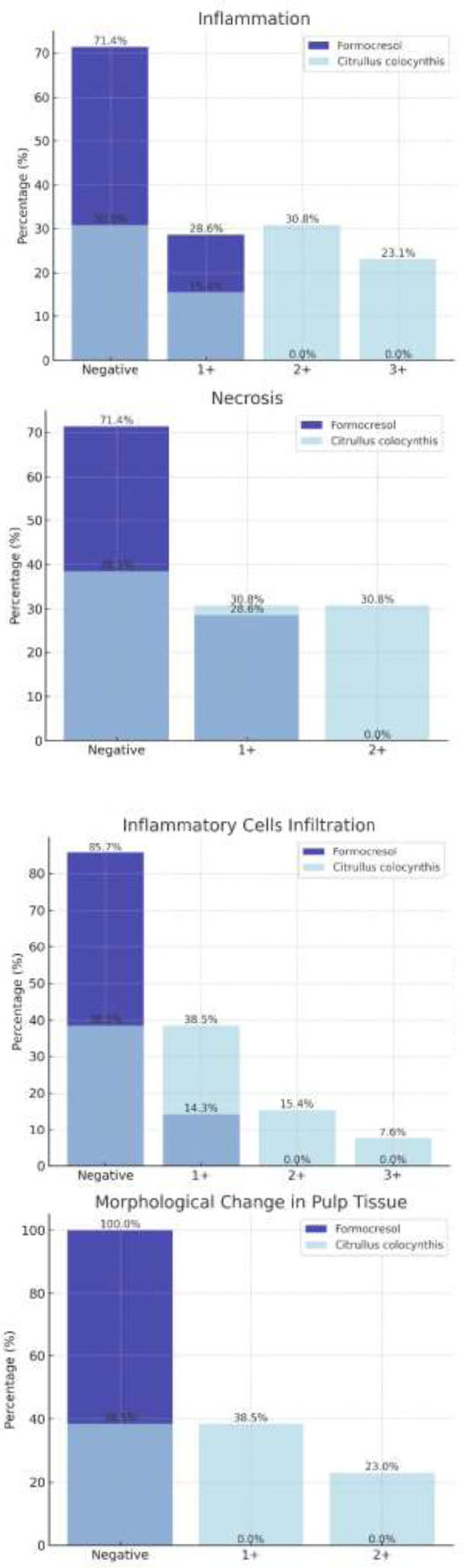
Comparison between groups based on studied parameters.
4. DISCUSSION
Plants and naturally-derived substances have long been integral to traditional medicine, with a multitude of therapeutic applications derived from a wide range of botanicals. The growing interest in plant-based medicines, especially as alternatives to synthetic antibiotics, emphasizes their potential importance. The fruit of Citrullus colocynthis, known for its anti-inflammatory and analgesic properties, was evaluated in this study for its efficacy in pulpotomy treatments and its impact on pulp tissue cells. Contrary to expectations based on its established medicinal uses, our findings indicated significant adverse cellular reactions in pulpotomy applications, highlighting a discrepancy between general anti-inflammatory benefits and specific clinical outcomes in dental treatments.
In our animal study, after preparing slides and conducting histopathological examinations [as detailed in the results], we observed a notable difference between the two intervention groups treated with formocresol and Citrullus colocynthis extract in terms of inflammation, infiltration of inflammatory cells, and morphological changes in pulp tissue cells. However, no significant difference was noted in the extent of tissue necrosis. Importantly, necrosis in the formocresol group was localized and associated with tissue fixation near the substance, whereas in the Citrullus colocynthis extract group, widespread necrosis followed tissue damage and inflammation. Additionally, control teeth were examined to ensure no pre-existing inflammation or disease, confirming the health of the pulp tissue in these samples.
The selection of rabbits as our experimental model was driven by several considerations. Although the use of rabbits in pulp therapy research is less common than other animals like dogs, cats, pigs, and monkeys, they offer notable advantages due to their economical upkeep and shorter lifespan. Rabbit teeth, particularly incisors, are of a suitable size for testing restorative procedures. Despite their teeth's continuous growth and wear, the results from applying pulp therapy materials on rabbit incisors align well with findings from numerous in vivo and in vitro studies, suggesting their suitability for short-term evaluations of new dental materials [25-29]. In a similar vein, Kayad et al. also utilized rabbit incisors in their study to evaluate dental materials, underscoring the methodological parallels between our approaches [24].
Exploring the antimicrobial efficacy of Citrullus colocynthis, studies by Barakian et al. and Tahmasebi et al. provide compelling insights. Barakian et al. focused on the ability of the extract to inhibit the growth of Streptococcus mutans and Lactobacillus acidophilus, pathogens associated with dental caries, without showing bactericidal properties [30]. Tahmasebi et al. extended this examination to include both antibacterial and antifungal properties against a broader spectrum of pathogens, demonstrating significant inhibitory and lethal effects [31]. While both studies confirm the broad-spectrum antimicrobial potential of Citrullus colocynthis, our research raises concerns about its direct application in pulpotomy due to observed adverse cellular responses, suggesting a discrepancy between antimicrobial efficacy and biocompatibility in direct pulp contact.
Similarly, Ghahramani et al. compared the antibacterial effectiveness of Citrullus colocynthis extract to calcium hydroxide against Enterococcus faecalis, finding the plant extract's performance commendable over a two-week period, with effects comparable to the traditional endodontic medicament [32]. This comparison underscores the potential of Citrullus colocynthis in microbial management but contrasts with the adverse effects observed in our pulpotomy study, highlighting the complexity of its clinical application.
Moreover, research by Alolofi et al. [33], which evaluates other natural substances in dental treatments [such as propolis and garden thyme], highlights both the potential and limits of using natural extracts. These studies provide a broader context within which our findings can be understood, suggesting that while natural substances like Citrullus colocynthis can offer significant benefits, their clinical application must be carefully evaluated to prevent adverse outcomes. This nuanced approach emphasizes the necessity for meticulous evaluation and optimization of natural extracts to ensure their efficacy and safety in clinical settings.
The study by Dehghani et al. on the toxicological effects of Citrullus colocynthis extract on pregnant mice highlighted significant systemic toxicity at higher concentrations, revealing concerns about the potential adverse effects on fetal viability and maternal survival [34]. This study underscores the critical need for careful dose management and further toxicity evaluation when considering Citrullus colocynthis for clinical applications.
By examining these studies together with our findings, it becomes clear that while Citrullus colocynthis exhibits significant therapeutic potential, its application in dental treatments requires meticulous evaluation to balance antimicrobial benefits against possible adverse cellular responses. Further investigations are essential to delineate the mechanisms of action and to establish safe and effective protocols for its use in dental therapies.
5. METHODOLOGY LIMITATIONS AND FUTURE IMPROVEMENTS
This study, while providing valuable insights into the use of Citrullus colocynthis as a pulpotomy medicament, is not without limitations. First, the sample size was constrained due to financial limitations, which prevented us from acquiring additional rabbits for the study. The ethics committee, recognizing the novelty of the subject and the lack of existing evidence for the treatment, only approved the use of eight rabbits, which restricted the number of subjects available for analysis. This limitation in sample size must be considered when interpreting the results.
Second, the choice of incisor teeth instead of molars was driven by practical considerations. Incisors offer better access to treatment, especially within the short duration of anesthesia, which is a critical factor when working with animal models. Additionally, several studies on pulpotomy or direct pulp capping treatments in rabbits have used incisors due to their size and accessibility, making them a standard choice in dental research with similar models. While we recognize that molars might provide more direct comparisons to human teeth, incisors were deemed the most feasible option given the constraints of the study.
A third limitation was the absence of Immunohistochemistry (IHC) staining. Due to financial constraints, we were unable to include IHC staining, which could have provided more detailed insights into inflammation and apoptosis mechanisms in the treated tissues. Although IHC staining was not incorporated in this study, we believe it is an important method that could enhance future studies on this subject, especially for confirming the mechanisms of cellular changes induced by Citrullus colocynthis.
Another key issue was the control group design. In this study, each rabbit served as its own control, with one tooth randomly treated with formocresol, given its long-established use and high success rates. The decision to use only one formocresol-treated tooth was based on the historical reliability of the material and ethical considerations to minimize animal use. Unfortunately, one of the formocresol samples was lost during the tissue cutting and staining process. For the Citrullus colocynthis treatment, each rabbit was treated with one or two teeth, depending on the cooperation of the animal during anesthesia. The split-mouth design was implemented to maximize the number of teeth treated with the extract, which resulted in an unequal number of samples across the two groups. This design choice was made to optimize the potential findings and increase the data for the experimental group.
CONCLUSION
Despite the antimicrobial properties and its ability to induce tissue necrosis, the ethanolic extract of Citrullus colocynthis is not recommended as a substitute for formocresol in pulpotomy treatments due to its high cellular toxicity. While it may alleviate acute symptoms in cases of severe pulpitis by inducing necrosis, its widespread necrotic effect and uncertain impact on surrounding tissues render its use inadvisable in these treatments.
SUGGESTIONS FOR FUTURE STUDIES
Future work could focus on evaluating alternative herbal medicaments for pulpotomy, with larger sample sizes and more robust experimental designs. Further, incorporating advanced techniques, such as immunohistochemistry could provide deeper insights into the biological mechanisms involved in pulp tissue response.
AUTHORS’ CONTRIBUTIONS
E.T.: Study conception and design; K.R.: Investigation; M.Y.: Visualization; S.H.: Original draft preparation; R.M.: and writing, reviewing, and editing. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| C.c. / C. | = Colocynthis Citrullus colocynthis |
| F. | = Formocresol |
| SPSS | = Statistical Package for the Social Sciences |
| S. mutans | = Streptococcus mutans |
| S. salivarius | = Streptococcus salivarius |
| E. coli | = Escherichia coli |
| S. aureus | = Staphylococcus aureus |
| L. acidophilus | = Lactobacillus acidophilus |
| C. albicans | = Candida albicans |
| mg | = Milligram |
| kg | = Kilogram |
| ml | = Milliliter |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval was obtained from the Baqiyatallah University of Medical Sciences' ethical committee [Ethics Code: IR.BMSU.BAQ.REC.1398.057], Iran prior to the initiation of research activities.
HUMAN AND ANIMAL RIGHTS
This study adheres to internationally accepted standards for animal research, following the 3Rs principle. The ARRIVE guidelines were employed for reporting experiments involving live animals, promoting ethical research practices. The animal experimentation was conducted according to the Guide for the Care and Use of Laboratory Animals.
AVAILABILITY OF DATA AND MATERIALS
All data generated or analyzed during this study are included in this published article.
ACKNOWLEDGEMENTS
The authors wish to express their gratitude to Shokri Dental Hospital for providing the facilities necessary to conduct the animal studies involved in this research. Their support was instrumental in the completion of this work.


