All published articles of this journal are available on ScienceDirect.
Comparative Evaluation of the Antimicrobial Efficacy of 20% Chlorhexidine, 3% Sodium Hypochlorite, and Dexamethasone Acetate with Thymol as a Root Canal Disinfectant against Enterococcus faecalis: An In-vitro Feasibility Study
Abstract
Introduction
Endodontic therapy aims to eliminate microbial infection from the root canal system. Enterococcus faecalis, known for its resistance and biofilm-forming capacity, presents significant challenges. This study evaluates the antimicrobial efficacy of 20% chlorhexidine (biosol), dexamethasone acetate with thymol (cresophene), and 3% sodium hypochlorite (NaOCl).
Methods
Eighty extracted uniradicular teeth were inoculated with E. faecalis and randomly assigned to four groups: Group I (biosol), Group II (cresophene), Group III (3% NaOCl), and Group IV (control). After irrigation, samples were incubated and assessed microbiologically after 48 hours to determine bacterial reduction.
Results
Group I (biosol) demonstrated the highest efficacy with a 90.15% reduction in bacterial load, closely followed by Group II (cresophene) at 89.85%. Group III (NaOCl) showed a 75.57% reduction. Group IV (control) had the highest bacterial persistence. Differences between Group I and Groups III/IV were statistically significant (p < 0.0001).
Discussion
The superior performance of biosol and cresophene highlights their potential as effective root canal disinfectants. The substantivity of biosol may help prevent recolonization, while the anti-inflammatory and antifungal properties of cresophene further support disinfection. Although NaOCl remains widely used, its limitations underscore the need for alternatives. Further research is warranted to assess safety, cytotoxicity, and clinical efficacy, particularly given the high concentration of chlorhexidine used.
Conclusion
Both biosol and cresophene demonstrated strong antimicrobial efficacy against E. faecalis, supporting their potential application in clinical endodontics. However, future clinical trials are necessary to validate these findings.
1. INTRODUCTION
The continuous integration of novel technologies and materials fundamentally drives the relentless pursuit of improved patient outcomes in dentistry. From advanced imaging to biomimetic restorative materials, innovation is the cornerstone of progress across all dental disciplines, including pediatric and adult endodontics. The preservation of natural primary and permanent teeth through effective endodontic therapy remains paramount, especially when confronted with pulpal or periapical diseases [1, 2].
Over the past decades, pediatric and adult endodontics have witnessed significant advancements, particularly in irrigation and chemical disinfection. Effective irrigation and chemical agents have been instrumental in enhancing treatment success. However, despite these strides, the intricate and often inaccessible anatomy of the root canal system presents persistent challenges. Literature highlights that traditional mechanical instrumentation alone cannot achieve thorough disinfection, leaving behind reservoirs for bacteria, biofilms, and debris [3]. This realization has underscored the critical role of irrigation in reducing microbial load and removing tissue remnants.
Recent technological developments, such as advanced microscopy and imaging, have further revealed the limitations of current techniques, demonstrating that certain areas, especially in oval canals and isthmuses, remain untouched by instrumentation [4]. This necessitates the exploration and development of novel chemical irrigants to complement mechanical preparation, aiming for more complete disinfection.
The ideal intracanal medicament must exhibit a multifaceted profile, including potent antimicrobial activity, stability in biological fluids, sustained effects, low surface tension for optimal penetration, biocompatibility, and minimal risk of tooth discoloration or adverse immune responses [5]. While various irrigants exist, each with distinct mechanisms of action, challenges remain in finding a solution that balances efficacy with safety. Concerns regarding cell damage from apical extrusion [6] emphasize the need for rigorous evaluation of new irrigants.
Chlorhexidine gluconate, a widely used irrigant, has demonstrated broad-spectrum antimicrobial activity [7]. However, the specific application of 20% chlorhexidine requires further investigation. Similarly, the use of steroids, such as dexamethasone acetate, in endodontics for inflammation and pain management is well-established [8], but their combination with agents like thymol (cresophene) as an irrigant is less explored.
The present study builds upon the existing literature by investigating the efficacy of two novel irrigants, 20% chlorhexidine (biosol) and dexamethasone acetate with thymol (cresophene), and comparing them to the gold standard, 3% sodium hypochlorite. Specifically, this study aims to address the knowledge gap regarding the antimicrobial effectiveness of these novel irrigants, offering a comparative analysis that goes beyond conventional approaches. The primary objective is to evaluate their efficacy in a controlled experimental setting, thereby contributing to developing evidence-based protocols for endodontic therapy. The null hypothesis postulates that there is no significant difference in the antimicrobial activity of these irrigants. By testing this hypothesis, we aim to identify the most effective irrigant, ultimately enhancing treatment outcomes and patient care. The significance of this study lies in its potential to expand the current understanding of root canal disinfection and provide clinicians with valuable insights for improved endodontic practice in pediatric and adult patients.
2. MATERIALS AND METHODS
This study aimed to compare the effectiveness of three different disinfection methods in reducing Enterococcus faecalis (E. faecalis), a common endodontic pathogen, in extracted human teeth. The study was approved by the ethical committee of Chhattisgarh Dental College and Research Institute, Rajnandgaon, India (Ref. no. CDCRI. 28/18). A total of 80 single-rooted permanent teeth, extracted for orthodontic and periodontal reasons, were selected for the study. The teeth were cleaned using a 5.2% NaOCl solution for 30 minutes to remove organic matter and then stored in a physiologic saline solution until the process began. The crowns were sectioned at the cementoenamel junction using a high-density diamond disc to obtain a uniform root canal length of 15mm. Access openings were made using endo access burs, and pre-coronal flaring was performed with Gates Glidden drills. The root canals were then prepared using the step-back technique (up to the #35 K file; 25 mm, Dentsply-Maillefer). After each instrumentation, the canals were irrigated using physiological saline solution and 5 mL of 3% sodium hypochlorite solution. EDTA gel was applied for 2-3 minutes to remove the smear layer.
2.1. Preparation of Inoculum
The bacterial strain E. faecalis (ATCC 29212) from HiMedia Labs, Pune, was used in this study. The cell density was adjusted to 9.7 x 108 cells/mL for each test group. Microbiological sampling was carried out before the application of medicaments. The test microorganisms were maintained on Brain Heart Infusion (BHI) agar slants at 40°C. An overnight BHI broth liquid culture of the organisms was prepared for each experiment. Microbiological sampling was conducted under sterile conditions to ensure that the teeth were free of bacterial contamination before the experiment. The teeth were autoclaved at 121°C for 20 minutes. The media was diffused in refined water and autoclaved at 15 lbs. pressure (121°C) for 15 minutes. The sterile teeth roots were inoculated with 10 µL of inoculum using a micropipette and then sealed with temporary cement after the placement of sterile cotton soaked with inoculum in the cervical access of the canal. The teeth were then incubated overnight at 37°C for 24 hours. The CFU of E. faecalis (ATCC 29212) was 500,000.
2.2. Procedure of Disinfection
The root canals of 80 teeth were dried with paper points and randomly divided into four groups, with 20 teeth in each group (Fig. 1). Group I received disinfection with 20% chlorhexidine (biosol). Group II received disinfection with dexamethasone acetate plus thymol (Cresophene). Group III received disinfection with 3% sodium hypochlorite, while Group IV served as the control group without any disinfection. Each group received 0.2 mL of the respective medicament, which was injected into the canals using a 26-gauge needle. To differentiate between the groups, a layer of colored enamel was applied to the teeth, and the apical foramen was sealed from the outside using nail varnish.
2.3. Assessment of CFU
After incubation for 48 hours, the root canals were cleaned and irrigated with saline, and then instrumentation was performed using a #45 file (25mm, Dentsply-Maillefer) to obtain dentinal shavings. The canals were again filled with physiological saline. Sterile paper points were placed in the canals for 30 seconds to collect the samples. The paper points were then placed into 1 mL of saline in test tubes, which were vigorously shaken and diluted to determine the colony-forming units per milliliter (CFU/mL). The survival of E. faecalis in the root canals of four experimental groups was quantified by determining colony-forming units (CFU). Aliquots from each sample were processed using the pour-plate method to estimate CFU/mL, and optical density (OD) at 600 nm was also measured. Survival fractions in each root specimen were calculated by counting the colonies on the experimental plates and dividing by the number of colonies from controls. Overall, this study aimed to evaluate the effectiveness of three different disinfection methods in reducing E. faecalis in extracted human teeth. The experimental design involved sterilizing, inoculating, and incubating the teeth with the test microorganisms, followed by applying the disinfection agents and incubating the specimens. The survival fractions of E. faecalis were then measured using colony-forming units and optical density (Fig. 2). The findings of this study could help inform clinicians when selecting disinfection agents for root canal treatment.
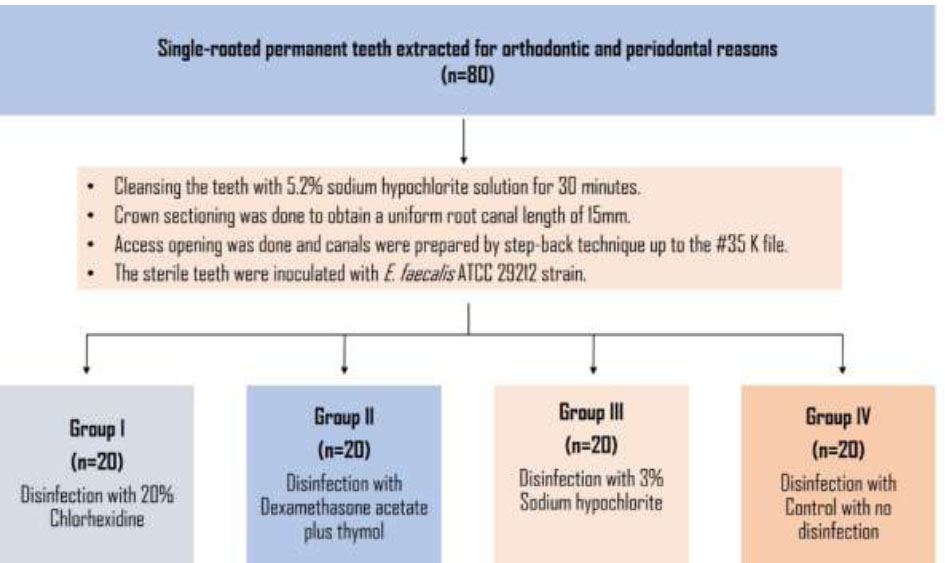
Flowchart illustrating the steps used in the material and methods.
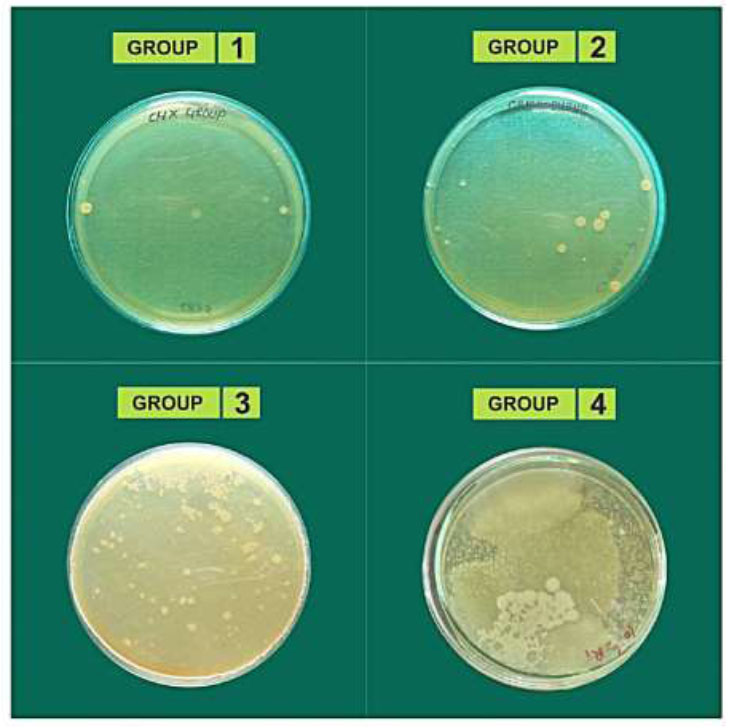
Colonies formed by E. faecalis.
2.4. Statistical Analysis
The data on counts of endodontic pathogens, i.e., E. faecalis, was obtained for 20 samples from each of the study groups. The mean and median values of the endodontic pathogen, i.e., E. faecalis, were obtained for all the study samples. The comparison of data across the groups was performed using a one-way analysis of variance (ANOVA) test. The pairwise comparison of counts between groups was carried out using Tukey’s post hoc test. The statistical significance was tested at 5%, and the analysis was performed using SPSS 20.0 ver. (SPSS Inc., Chicago, IL, USA).
3. RESULTS
3.1. Distribution of E. faecalis among Study Groups
Table 1 presents the distribution of E. faecalis counts across the study groups, offering a visual representation of our investigation. Each group's E. faecalis count was measured, and the relative standard deviation was calculated to assess variability. Group I had the smallest E. faecalis count, with a mean of 7.94 CFU/ml and a relative standard deviation of 2.26, indicating lower bacterial presence on average than the other groups. Group II had a mean count of 7.97 CFU/ml and a relative standard deviation of 1.37, suggesting relatively less variability than Group I. Group III recorded a mean count of 8.37 CFU/ml and a relative standard deviation of 0.51, showing the least variability among the groups. Group IV had the highest mean count of E. faecalis, with 9.28 CFU/ml and a relative standard deviation of 0.91. Significant differences in E. faecalis counts were observed among all groups (p < 0.0001), indicating experimental manipulation as the likely cause. The lowest mean count of E. faecalis was found in Group I, with significant differences noted among all groups. Relative standard deviation values provided additional insight into variability within each group.
| Groups | E. faecalis Count | Significance | |
|---|---|---|---|
| Mean ± SD | F - value | p-value | |
| Group I | 7.94 ± 2.26 | 587.3 | < 0.0001 |
| Group II | 7.97 ± 1.37 | ||
| Group III | 8.37 ± 0.51 | ||
| Group IV | 9.28 ± 0.91 | ||
3.2. Comparison of E. faecalis within Study Groups
Table 2 presents the results of a study comparing the efficacy of various disinfectants in reducing E. faecalis counts. Mean counts and standard deviations were calculated for each group to assess differences. Group I had a mean count of 7.94 ± 2.26, and Group II had 7.97 ± 1.37, with a non-significant p-value of 0.72, indicating no difference. Group III (8.37 ± 0.51) had significantly fewer counts than Group II (p < 0.0001), suggesting the superiority of biosol over NaOCl. Group IV (9.28 ± 0.91) had significantly more counts than Group I (p < 0.0001), implying that the disinfectant of Group IV was less effective. Group II (7.97 ± 1.37) had significantly fewer counts than Group IV (p < 0.0001), indicating the superiority of cresophene over NaOCl. Biosol was more effective than NaOCl, and Group IV had the highest E. faecalis count. Standard deviation values offered insights into result variability within groups.
| Groups | E. faecalis | |||
|---|---|---|---|---|
| Mean ± SD | p-value | |||
| Group I | vs. | Group II | 7.97 ± 1.37 | 0.7228 |
| Group III | 8.37± 0.51 | < 0.0001 | ||
| Group IV | 9.28 ± 0.91 | < 0.0001 | ||
| Group II | vs. | Group III | 8.37 ± 0.51 | < 0.0001 |
| Group IV | 9.28 ± 0.91 | < 0.0001 | ||
| Group III | vs. | Group IV | 9.28 ± 0.91 | < 0.0001 |
3.3. Comparing Disinfectant Efficacy against E. faecalis
Table 3 presents the outcomes of a study evaluating the efficacy of diverse disinfectants in decreasing E. faecalis counts in root canals. The log reductions in the bacterial count were calculated as Log Reduction=log10 (A/ B), where ‘A’ denotes the number of viable microorganisms before treatment and ‘B’ denotes the number of viable microorganisms after treatment. The investigation comprised four groups, each exhibiting a distinct mean count of E. faecalis. Group IV notably displayed a significantly higher count compared to Group III. Additionally, the study reported mean reductions in E. faecalis count for each group; Group I achieved a mean reduction of 90.15 ± 4.78%, Group II achieved 89.85 ± 2.77%, and Group III achieved 75.57 ± 2.46%. Notably, 20% CHX demonstrated the most substantial reduction, while 3% sodium hypochlorite exhibited the least reduction. These findings underscore the significant influence of disinfectant type and concentration on reducing E. faecalis count in root canals, with cresophene proving more effective than NaOCl and 20% CHX demonstrating the highest reduction.
| Groups | Reduction in E. faecalis Count (%) |
|---|---|
| Group I | 90.15% |
| Group II | 89.85% |
| Group III | 75.57% |
4. DISCUSSION
The use of antimicrobial medicaments in endodontic treatment is pivotal for the success of root canal therapy by eliminating microorganisms from the root canal space [9]. However, persistent periapical lesions caused by microorganisms like E. faecalis pose challenges due to their biofilm formation, which shields them against traditional treatment methods and resistance to antimicrobial agents [10, 11]. Additionally, the emergence of multidrug-resistant E. faecalis strains underscores the need for effective intraradicular disinfectants [12]. In this study, we evaluated the efficacy of different root canal disinfectants using the E. faecalis 29212 strains. One disinfectant, chlorhexidine, well-known for its broad-spectrum antibacterial properties, exhibited promising results due to its immediate antimicrobial action, biocompatibility, and mode of action involving Sortase A protein interference [13-17]. We used a higher concentration, i.e., 20% chlorhexidine (biosol), known for enhanced bactericidal activity and stability at a broader pH range [18, 19]. Chlorhexidine at concentrations above 2%, combined with Cetrimide (CTR), can eliminate E. faecalis similar to 2.5% NaOCl [20]. However, lower concentrations (0.2%) may exhibit a bacteriostatic effect by inhibiting membrane function [21, 22]. Despite limited literature on 20% chlorhexidine, we chose it for its potential efficacy. The long-lasting substantivity of chlorhexidine refers to its ability to bind to dentine and exert a prolonged antimicrobial effect, making it valuable in endodontics, where disinfection of the root canal system is crucial [13, 14, 23]. One of the main arguments favoring CHX is its ability to bind to dentine and exert a prolonged antimicrobial effect (substantivity), which may prevent bacterial recolonization after root canal treatment. In cases of severe root canal infection, particularly those exhibiting sinus tracts, purulence, or requiring retreatment, final irrigation with 2% chlorhexidine is recommended [24].
Moreover, the present study explored cresophene, a combination of dexamethasone acetate and thymol, which achieved an 89.95% reduction in E. faecalis counts, comparable to biosol, suggesting its effectiveness in root canal disinfection. Corticosteroids in cresophene contribute to inflammation reduction and membrane stabilization, reducing post-operative pain [24]. Thymol, an antifungal agent, enhances disinfection by altering membrane permeability and has demonstrated antimicrobial activity against E. faecalis [25-27]. Camphor oil, containing cinnamaldehyde and other antimicrobial compounds, shows promise as an alternative root canal disinfectant [28, 29]. Still, further research is required to evaluate its effectiveness. Sodium hypochlorite (NaOCl), a common root canal irrigant with broad-spectrum antimicrobial activity, is effective but carries the risk of adverse effects, including tissue damage, pain, and tissue discoloration [30-39]. In our study, lower concentrations of sodium hypochlorite (3% NaOCl) resulted in a 75% reduction in Enterococcus faecalis counts. However, this concentration has limitations and may lead to potential adverse effects. As a result, alternative disinfectants, such as 20% chlorhexidine and cresophene, may provide additional benefits in root canal therapy, meeting the demand for effective and safe intraradicular disinfectants [18, 19].
4.1. Study Limitations and Scope for Further Research
While the present in-vitro study demonstrates the potential efficacy of novel irrigants (20% chlorhexidine and dexamethasone acetate with thymol) against E. faecalis, it presents several limitations that necessitate cautious interpretation and guide future research directions. Firstly, the inherent constraints of an in vitro model limit its ability to fully replicate the complex and dynamic environment of the clinical setting. Consequently, direct extrapolation of these findings to in-vivo conditions should be approached with prudence.
Secondly, the study's focus on E. faecalis as the sole target microorganism, while clinically relevant, does not reflect the polymicrobial nature of endodontic infections. Future investigations should expand the microbiological spectrum to include a wider range of bacterial species commonly encountered in root canal systems, thus providing a more comprehensive assessment of disinfectant efficacy.
Thirdly, the use of 20% chlorhexidine, a concentration exceeding typical clinical applications, raises concerns regarding its safety profile. Potential adverse effects, such as tissue damage and allergic reactions, warrant further evaluation. Future studies should explore varying concentrations of chlorhexidine and investigate alternative intracanal medicaments to optimize both efficacy and safety.
Furthermore, the absence of cytotoxicity assessments in this study represents a critical limitation. Given the potential for these disinfectants to interact with periapical tissues, future research must incorporate comprehensive cytotoxicity evaluations to ensure their biocompatibility and safety for clinical use.
To advance our understanding of root canal disinfection and translate these in-vitro findings into clinically relevant protocols, we recommend the following:
- Expanding the microbiological evaluation to include a broader spectrum of microorganisms relevant to endodontic infections.
- Performing comprehensive cytotoxicity assessments to determine the biocompatibility of these disinfectants.
- Conducting well-designed clinical trials to validate the efficacy and safety of these disinfectants in vivo.
Future research addressing these limitations and recommendations is essential for enhancing our understanding of root canal disinfection and ultimately improving treatment outcomes in endodontic practice.
CONCLUSION
Within the limitations of this in-vitro feasibility study, 20% chlorhexidine (biosol) and dexamethasone acetate with thymol (cresophene) demonstrated effective disinfection of root canals. Notably, the substantivity of 20% chlorhexidine suggests its potential for prolonged antibacterial effects. Nevertheless, these findings require validation through in-vivo investigations. Future research should incorporate comprehensive cytotoxicity evaluations to ensure their biocompatibility and safety for clinical use.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: R.M.S.: Writing the Paper,T.G.: Data Collection, S.S.Y. , P.A., K.I.A. : Study Concept or Design, V.B.D.: Data Analysis or Interpretation. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| E. faecalis | = Enterococcus faecalis |
| CFU | = Colony Forming Unit |
| CHX | = Chlorhexidine |
| BHI | = Brain Heart Infusion |
| OD | = Optical Density |
ETHICAL STATEMENT
This study was approved by the ethical committee of Chhattisgarh Dental College and Research Institute, Rajnandgaon, India (Ref. no. CDCRI. 28/18).
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Government V.Y.T.P.G. Autonomous College (College of Arts and Sciences) and Dr. Dhananjay Raje, Head, Data Analytics Division at MDS Bio-Analytics Pvt. Ltd. Nagpur.


