All published articles of this journal are available on ScienceDirect.
Alveolar Ridge Augmentation using Customized Zirconia Membranes: A Systematic Review
Abstract
Background
Since the inception of intraosseous implants, the significance of alveolar bone volume has become crucial in formulating treatment plans for dental implants. Various barrier membranes have been extensively employed in alveolar ridge reconstruction, highlighting their efficacy. Recent advancements include the fabrication of customized barrier membranes using multiple materials, with titanium and zirconia being prominent choices. The objective of this study was to conduct a comprehensive review of all clinical studies, case reports, and case series that utilized customized zirconia membranes for alveolar ridge augmentation.
Methods
An electronic literature search was performed to find relevant clinical studies, case reports, and case series published in English up to August 2024. The following keywords used in the search were customized zirconia membrane, alveolar ridge augmentation, Guided bone regeneration, zirconia sheet, ceramic sheet, and Personalized membrane. The quality assessment was conducted using the Joanna Briggs Institute (JBI) critical appraisal checklist specific to each type of study.
Results
The electronic search initially yielded 539 articles. Following deduplication, 263 unique articles remained. Subsequent manual screening of titles and abstracts led to the exclusion of 250 articles, resulting in 13 remaining articles. After conducting a thorough full-text assessment of these 13 articles to verify adherence to the inclusion/exclusion criteria, 6 articles were further excluded, leaving a total of 7 articles included in this review.
Conclusion
While all studies included in this review were case series or case reports, customized zirconia membranes have demonstrated effectiveness in the literature for alveolar ridge augmentation, facilitating the placement of dental implants in all cases with relatively low complication rates, particularly membrane exposure. There is currently no literature evidence supporting the superiority of zirconia over other materials used in customized fabrication, nor is there evidence of superior design or preferred bone grafting under these membranes.
1. INTRODUCTION
Since the discovery of osseointegration and the development of intraosseous dental implants, the significance of alveolar bone in formulating treatment strategies for tooth replacement has become more prominent. The quantity of alveolar bone plays a crucial role, closely intertwined with the presence of teeth [1]. Research conducted over the years has consistently demonstrated inevitable bone resorption in both horizontal and vertical dimensions following tooth loss [2]. In addition to tooth loss, factors such as trauma, periodontal diseases, tumors, cysts, and other pathological conditions can also contribute to bone loss [2].
Researchers have made significant advancements in horizontal and vertical alveolar ridge augmentation techniques over the past few decades [3, 4]. These techniques are typically categorized into four main approaches: guided bone regeneration, bone block grafting, distraction osteogenesis, and osteoperiosteal flaps [5, 6]. Additionally, methods such as bone expansion and splitting are utilized for horizontal augmentation [7], while external and transalveolar sinus lift techniques are employed for vertical augmentation [8].
Guided bone regeneration is a widely used technique in oral and maxillofacial surgery clinics [9]. The fundamental principle involves creating a space between the host bone and soft tissues, which is then preserved using space-maintaining devices. Subsequently, the defect is filled with living bone [10]. An essential aspect of the success of guided bone regeneration is inhibiting the growth and proliferation of non-osseous cells in the targeted bone regeneration area. Barrier membranes are commonly utilized for this purpose [11].
The most frequently employed membranes in guided bone regeneration are resorbable collagen membranes and non-resorbable PTFE membranes [12]. These membranes possess excellent barrier properties that effectively prevent the infiltration of undesired cells into the bone defect. However, their space-maintaining capability is restricted, making them unsuitable for extensive bone augmentation procedures [13]. In such cases, titanium meshes and PTF titanium-reinforced membranes have been utilized as alternatives to the aforementioned membranes for comprehensive bone augmentation procedures [13-15]. Despite their established clinical success and predictability, these techniques have several drawbacks, such as the requirement for customization to fit the shape and size of the bone defect, extended surgical duration, relatively elevated exposure rates leading to procedural failure, limited barrier effectiveness of titanium meshes, and lastly, their considerable cost [13-15].
In recent times, 3D printing techniques have been integrated into the realm of bone regeneration through both additive and subtractive methodologies [16]. Initially, models were printed to replicate the patient's bone defect area, facilitating the pre-conditioning of titanium meshes, thereby streamlining the process. Subsequently, researchers utilized 3D printing or milling to create scaffolds from various materials ready for direct clinical implementation on the host site. Lastly, individualized container shells, often referred to as customized membranes, were fabricated using 3D printing or milling techniques [17].
Customized membranes can be described as space-maintaining devices tailored and produced to suit a specific bone defect aimed at rehabilitation of the area with dental implants. Initially, customized membranes were printed from titanium, the most extensively acknowledged and utilized metal in the domain of oral and maxillofacial surgery [18, 19].
Customized titanium meshes have demonstrated superior success rates compared to prefabricated meshes in terms of reducing complications, enhancing clinical outcomes, promoting bone gain, and streamlining the surgical process [16, 18]. Although titanium meshes have proven effective, their high cost has led researchers to explore zirconia as a potential alternative material for producing customized zirconia membranes to augment the alveolar ridge [20-26]. This systematic review seeks to assess the efficacy of customized zirconia membranes in alveolar bone augmentation.
The primary objective of this study is to offer a comprehensive review of various facets associated with the utilization of customized zirconia membranes, encompassing:
- Equipment and software employed for designing and fabricating customized zirconia membranes.
- Various design forms.
- Types of bone grafting materials utilized beneath these membranes.
- Assessment of bone gain, bone density, and histological analysis of newly formed bone.
- Identification of potential complications, their underlying causes, and strategies for prevention.
2. METHODS
The present systematic review is reported in accordance with the guidelines for the Transparent Reporting of Systematic Reviews and Meta-analyses [27, 28] following the 2020 Prisma Guidelines [29]. The study protocol was prospectively registered on PROSPERO (CRD42024588058).
2.1. Focused Questions
The focused question was set according to the PICO population or problem (P), intervention (I), comparison (C), and outcome (O) strategy as follows:
2.2. Search Strategy
An electronic search was undertaken in PubMed/Medline, Scopus, Web of Science, and Science Direct databases until August 2024. The following terms were used in the search strategies: Customized zirconia membrane, alveolar ridge augmentation, Guided bone regeneration, zirconia sheet, ceramic sheet, and Personalized membrane. A manual search of dental implants-related-journals was also performed. The reference lists of the identified studies and the relevant reviews on the subject were checked for possible additional studies.
2.3. Inclusion and Exclusion Criteria
2.4. Screening Methods
For the study selection process, these steps were followed:
2.5. Data Extraction
The following data were extracted:
2.5.1. Study Identification
Author(s), Year of publication, Title of the study, Journal, Study type, and Focused questions of the study.
2.6. Quality Assessment
The quality assessment of the case reports and case series was conducted using the Joanna Briggs Institute (JBI) critical appraisal checklist specific to each type of study. This assessment was carried out by two reviewers (ZA and AK), and any discrepancies were resolved through discussion with a third reviewer (WK).
3. RESULTS
3.1. Inclusion and Exclusion of Articles
The electronic search initially yielded 539 articles. Following deduplication, 263 unique articles remained. Subsequent manual screening of titles and abstracts led to the exclusion of 250 articles, resulting in 13 remaining articles. After conducting a thorough full-text assessment of these 13 articles to verify adherence to the inclusion/exclusion criteria, 6 articles were further excluded, leaving a total of 7 articles included in this review [20-26]. A flow diagram reporting the screening and selection of studies is presented in Fig. (1).
| Article No. | Authors/Refs | Year | Journal | Type |
|---|---|---|---|---|
| 1 | Malmstrom et al. [20] | 2016 | International Journal of Oral and Maxillofacial Surgery | Case series |
| 2 | Hofferber et al. [22] | 2018 | The Journal of Implant & Advanced Clinical Dentistry | Two case report |
| 3 | Heikal et al. [23] | 2020 | Al-Azhar Journal of Dental Science | Case series |
| 4 | Mandelli et al. [24] | 2021 | Journal of Dentistry | Case series |
| 5 | Souidan et al. [21] | 2023 | Alexandria Dental Journal | Case series |
| 6 | Joseph et al. [25] | 2024 | South Asian Research Journal of Oral and Dental Sciences | Case Report |
| 7 | Albash et al. [26] | 2024 | The Open Dentistry Journal | Case report |
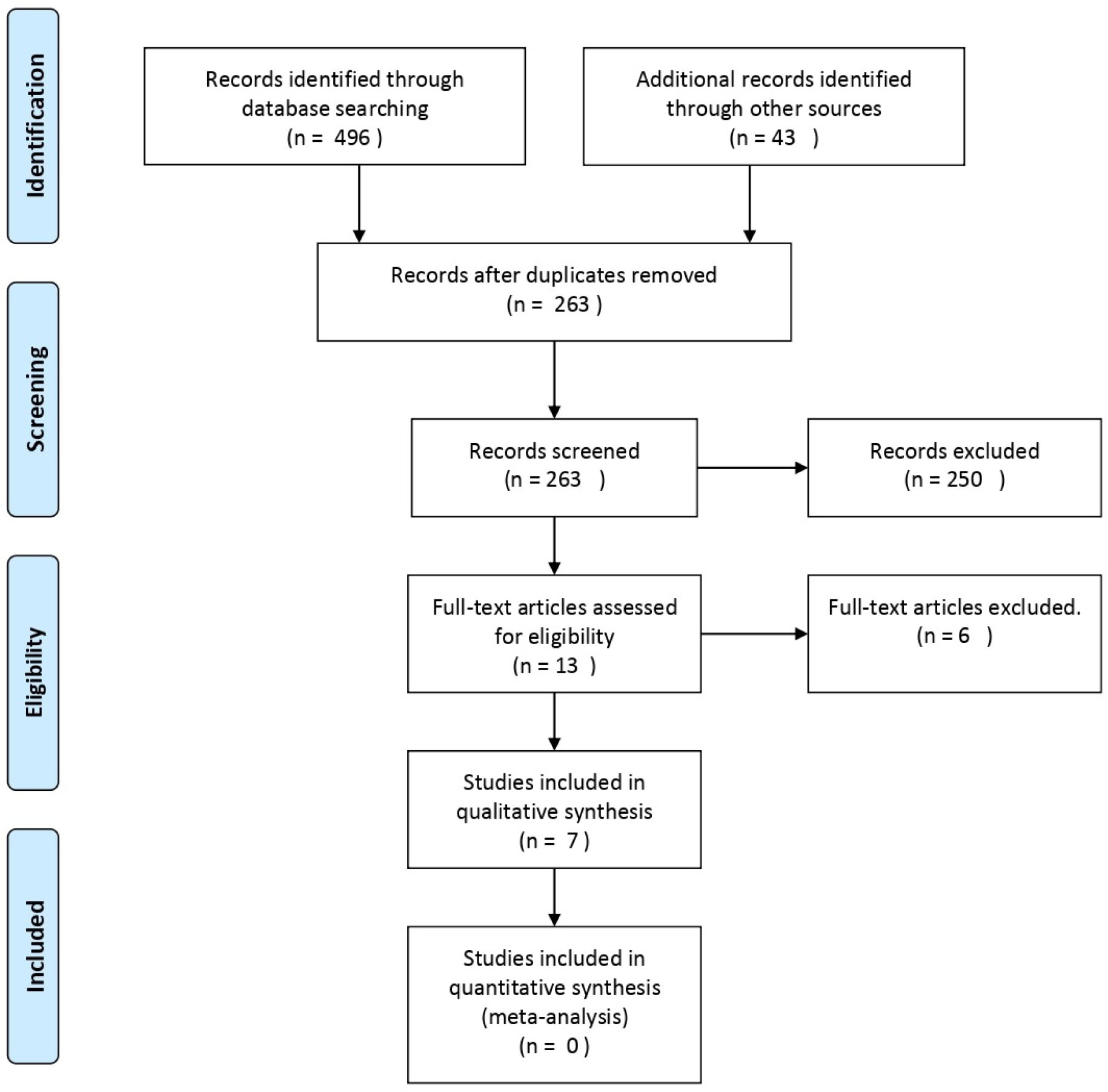
A Flow diagram of study selection process.
3.2. Description of the Selected Studies
Between the years 2016 and 2024, four case series [20, 21, 23, 24] and three case reports [22, 25, 26] were chosen for inclusion (Table 1). These studies collectively employed a consistent surgical approach for addressing horizontal, vertical, or combined bone defects in various jaw regions, either with or without bone grafts. Notably, none of the studies included comparison groups. The common technique utilized across all studies involved the application of computer-aided design and manufacturing (CAD/CAM) for the creation of customized (personalized) zirconia membranes (Fig. 2).
3.3. Quality Assessment of the Studies
The findings from the JBI critical appraisal checklist for case reports and case series can be found in (Table 2). In general, the quality of the studies included ranged from moderate to good. Each of the case reports met 6 out of the 8 quality criteria or more, while all case series met 6 out of the 10 quality criteria or more.
3.4. Patient Features
In this review, a total of 30 patients (comprising 11 males and 19 females) with an average age of 45.83 ± 16.35 years were included. These individuals underwent a collective total of 40 bone augmentation procedures using CZM (Table 3).
3.5. Description of Membranes
The membranes utilized in the included articles were predominantly designed similarly, albeit with distinct software and milling processes. They were custom-designed after importing the patient's Digital Imaging and Communications in Medicine (DICOM) files into the respective processing software. Through these software tools, the membrane was digitally crafted, milled, and then sintered using specialized equipment. Each membrane was uniquely dimensioned to precisely fit the specific area of the bone defect in each patient (Table 4).
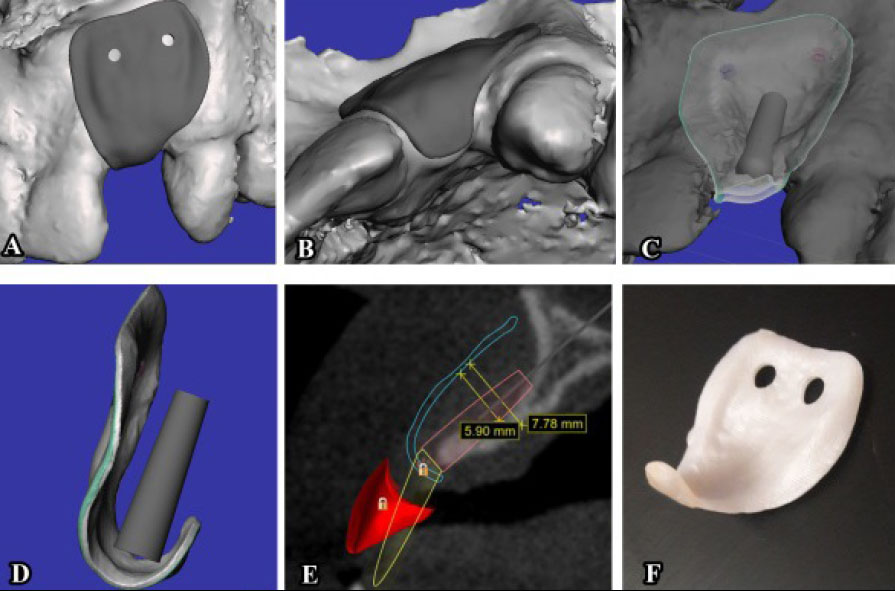
Design of CZM at a virtual model based on DICOM format file [r].
| Authors/Year | Type | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | Total of Yes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malmstrom 2016 [20] | Case series | No | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 6/10 |
| Hofferber 2018 [22] | Case report | No | Yes | Yes | Yes | Yes | Yes | No | Yes | - | - | 6/8 |
| Heikal 2020 [23] | Case series | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7/10 |
| Mandelli 2021 [24] | Case series | No | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 6/10 |
| Souidan 2023 [21] | Case series | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7/10 |
| Joseph 2024 [25] | Case report | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | - | - | 7/8 |
| Albash 2024 [26] | Case report | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | - | - | 7/8 |
| Article No. | Authors/Refs. | Number of Patients | Number of Procedures | Age | Gender | |
|---|---|---|---|---|---|---|
| - | Male | Female | ||||
| 1 | Malmstrom [20] | 3 | 4 | 50.66 ±18.03 | 1 | 2 |
| 2 | Hofferber [22] | 2 | 2 | 37 ± 1.41 | 1 | 1 |
| 3 | Heikal [23] | 7 | 14 | 55.86±4.6 | 3 | 4 |
| 4 | Mandelli [24] | 7 | 9 | 63.1 ± 6.2 | 2 | 5 |
| 5 | Souidan [21] | 10 | 10 | 29.60 ± 6.83 | 4 | 6 |
| 6 | Joseph [25] | 1 | 1 | 25 ± 0 | 1 | 0 |
| 7 | Albash [26] | 1 | 1 | 20 ± 0 | 0 | 1 |
| - | Total | 31 | 41 | 45.83 ± 16.35 | 12 | 19 |
| Authors/Refs. | Thickness | Milling Machine | Milling Machine Trading Name | Zirconia Disc | Sintering Temperature | Software | Sterilization | Porosity | Screws Number |
|---|---|---|---|---|---|---|---|---|---|
| Malmstrom 2016 [20] | 0.7 | 5-axis | Dechel-Maho, DMU 60, Deckel Maho, Pfronten, Germany | TZ3YSEB; Tosoh | 1450°C | - | Autoclave 134°C | Non-porous | 1 |
| Hofferber 2018 [22] | - | 5-axis | CORiTEC 350i, imesicore GmbH | Zenostar MO, Ivoclar Vivadent | - | Materialise, Leuven, Belgium | - | Porous | 1 |
| Heikal 2020 [23] | - | 5-axis | Ceramill motion 2 5x, Amann Girrbach, Lichtenstein | - | - | - | - | Non-porous | Multiple |
| Mandelli 2021 [24] | 0.4–0.5 | 5-axis | M1, Zirkonzahn, Gais, Italy | Prettau, Zirkonzahn, Gais, Italy | - | Exocad GmbH, Germany | Autoclave 134°C for 18 min | Non-porous | Multiple |
| Souidan 2023 [21] | 0.7 | 5-axis | Roland DWX 50®, Japan | Dental Direct®, Germany | 1500°C | Blueskybio, Illinois, US | Autoclave 134°C | Non-porous | Multiple |
| Joseph 2024 [25] | - | 5-axis | PrograMill PM7, Ivoclar, Schaan, Liechtenstein | Ivoclar Vivadent | - | MeshMixer, Autodesk, San Rafael, CA | - | Non-porous | Multiple |
| Albash 2024 [26] | 0.5 - 1 | 5-axis | Roland DWX 50®, Japan | Dental Direct®, Germany | 1500°C | Exocad GmbH, Germany | - | Non-porous | 1 |
Certain authors mentioned that they designed the dimensions and morphology of the virtual bone with consideration for future prosthetics (prosthetically designed guided), ensuring that subsequent dental implants could be placed to align appropriately with the future prosthesis. However, not all authors detailed this aspect in their reports.
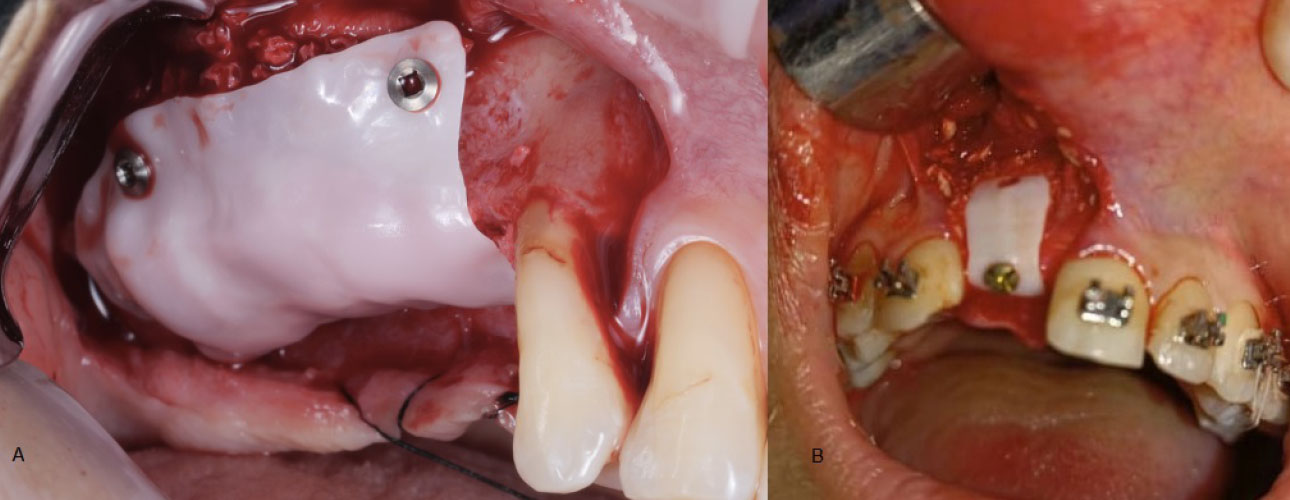
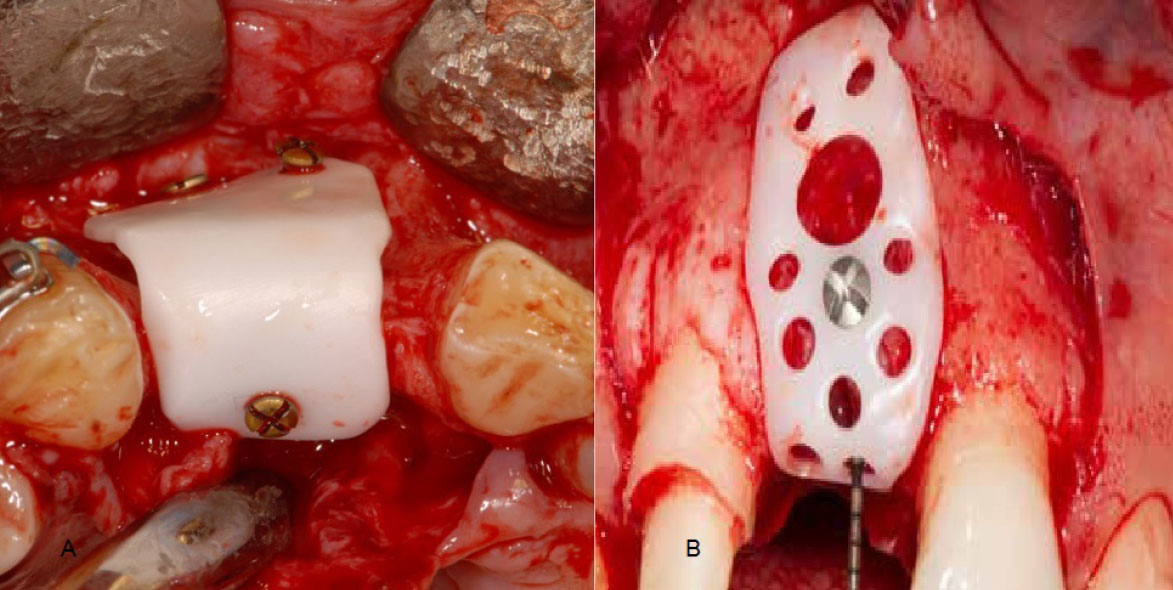
In the design process, special holes were created for fixation screws. Some authors noted that a single fixation screw proved adequate to stabilize the membrane and prevent movement. Conversely, in cases of more extensive bone defects, other authors opted for multiple fixation screws (Fig. 3). While the thickness of the membranes varied across studies, it generally did not exceed one millimeter in most instances (Table 4).
Except for the membranes employed in the Hofferber et al. [22] study, which were porous, all other membranes used in the reviewed cases were nonporous (Fig. 4 and Table 4).
Across all studies, the CZMs were designed as a single continuous piece, except Joseph et al. [25] study, where the CZM was divided into two separate pieces—a buccal piece and a palatal piece (Fig. 5).
3.6. Bone Graft Material and Defect Features
This review encompassed three specific regions of the jaws: the anterior region of the maxilla, where 13 procedures were conducted; the posterior region of the maxilla, where 9 procedures took place; and the posterior region of the mandible, where 18 procedures were carried out (Table 4).
Various bone graft materials were employed across the studies included in this review. The most prevalent choice was xenogeneic (bovine) bone graft, utilized in 17 procedures, constituting 42.5% of the cases. A combi- nation of graft materials was employed in 10 procedures, accounting for 25% of the total. Additionally, two cases utilized allografts solely, while one case utilized an autograft harvested from the same recipient site. Notably, in 10 procedures (25%), no bone graft materials were utilized (Table 4).
Bone defects were categorized as either vertical only, horizontal only, or both vertical and horizontal. Out of the included studies, five reported the specific type of bone defect addressed, involving a total of 23 patients and 31 procedures. Only one study did not provide this information, encompassing 7 patients and 9 procedures. Upon review, the distribution of bone defects managed in the included studies was as follows: 4 cases of vertical defects only, 8 cases of horizontal defects only, and 19 cases involving both horizontal and vertical defects (Table 5).
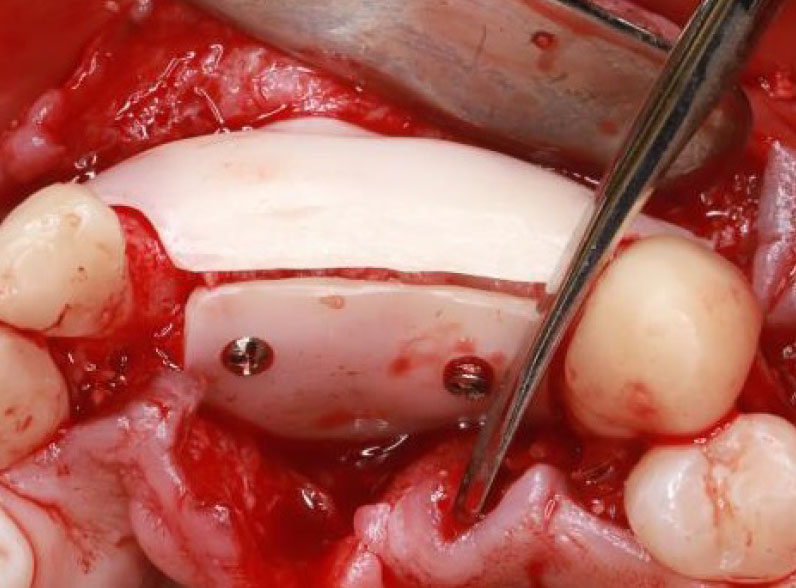
Two-piece membrane design [25].
| Authors/Refs. | Region | Defect | Bone Graft | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior Maxillary | Posterior Maxillary | Posterior Mandibular | Vertical | Horizontal | Horizontal & Vertical | Auto | Allo | Xeno | Mix | No Bone Graft | |
| Malmstrom [20] | 1 | 1 | 2 | 4 | 0 | 0 | 1 | 0 | 0 | 0 | 3 |
| Hofferber [22] | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| Heikal [23] | 0 | 0 | 14 | 0 | 0 | 14 | 0 | 0 | 7 | 0 | 7 |
| Mandelli [24] | 0 | 8 | 1 | - | - | - | 0 | 0 | 0 | 9 | 0 |
| Souidan [21] | 10 | 0 | 0 | 0 | 6 | 4 | 0 | 0 | 10 | 0 | 0 |
| Joseph [25] | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Albash [26] | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Total | 13 | 9 | 18 | 4 | 8 | 20 | 1 | 3 | 17 | 10 | 10 |
| Authors/Refs. | Bone Gain | Bone Density | |||
|---|---|---|---|---|---|
| Vertical | N | Horizontal | N | ||
| Malmstrom [20] | 2.9 ± 1.126 | 3 | 0 | 0 | - |
| Hofferber [22] | 3 | 1 | 3.5 ± 0.353 | 2 | - |
| Heikal [23] | 2.44 ± 0.92 | 14 | 1.76 ± 0.55 | 14 | 465.9 ± 367.9 |
| Mandelli [24] | - | - | - | - | - |
| Souidan [21] | 5.58 ± 1.26 | 4 | 2.18 ± 1.05 | 10 | 806.39 ± 206.76 |
| Joseph [25] | - | - | - | - | - |
| Albash [26] | 0 | 0 | 5.55 | 1 | - |
3.7. Bone Gain & Bone Density
The extent of horizontal or vertical bone gain differed among studies, yet all studies indicated that the resulting bone growth was enough to accommodate appropriate dental implants. In their research, Heikal et al. [23] (The sole study that compared bone gain with and without bone graft) observed statistically significant disparities in vertical and horizontal bone gain between the bone graft group and the membrane-only group without bone graft (Table 6).
Many studies noted the favorable quality of the newly formed bone (without assessing bone density), resulting in satisfactory insertion torque for dental implants in the grafted region. Only two studies evaluated bone density using the Hounsfield scale. Souidan et al. [21] found that the average bone density of the newly formed bone was 806.39 ± 206.76, whereas Heikal et al. [23] reported a mean bone density of 465.9 ± 367.9 across their entire sample. Heikal [23] further highlighted significant disparities between the two study groups (with and without graft), with the bone density of the formed bone in the graft group measuring 796.1 ± 181.9, compared to 135.7 ± 76.30 in the membrane-only group without graft (Table 6).
3.8. Histological Analysis
The authors of the majority of studies included in this review noted the presence of a soft tissue layer between the membrane and the newly formed bone (Fig. 6). Three of the studies conducted histological analysis on this soft tissue layer and observed it to be a connective tissue resembling the periosteum, with no signs of inflammatory response. Histological examination of bone biopsies revealed typical osteoblast and osteoclast activity, along with dilated vessels characterized by thin walls.
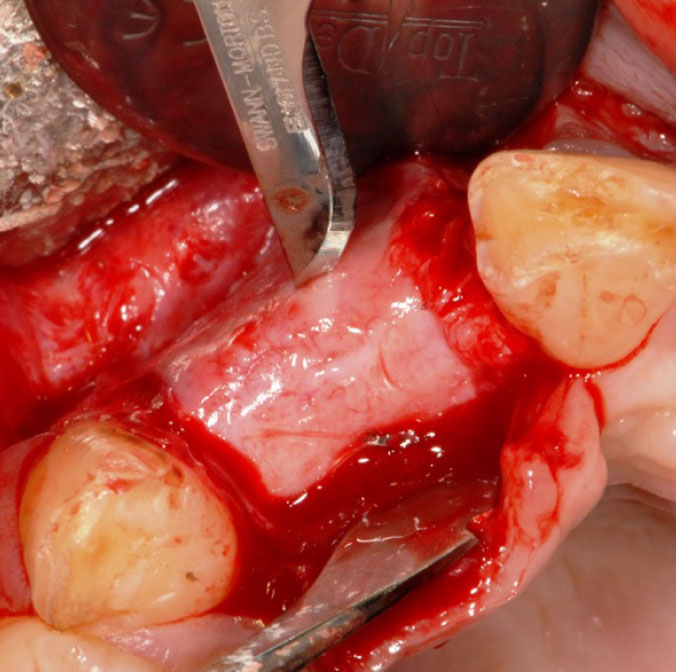
Pseudo-periosteum on the surface of the newly formed bone [20].
| Authors/Refs. | Number of procedures | Premature membrane exposure | Week | Possible causes | Loss of bone graft materials | Augmentation failure |
|---|---|---|---|---|---|---|
| Malmstrom [20] | 4 | 0 | - | - | 0 | 0 |
| Hofferber [22] | 2 | 0 | - | - | 0 | 0 |
| Heikal [23] | 14 | 0 | - | - | 0 | 0 |
| Mandelli [24] | 9 | 1 | 4 | Suboptimal membrane design with sharp edges. | 0 | 0 |
| Souidan [21] | 10 | 9 | 5.80 ± 5.53 | Suboptimal membrane design. Absence of periosteal sutures. Palatally based incision line. Excessive palatal extension. Anterior maxillary region. |
1 | 0 |
| Joseph [25] | 1 | 1 | 13 | - | 0 | 0 |
| Albash [26] | 1 | 0 | - | - | 0 | 0 |
2.9. Complications
The most frequently reported complication by the authors was premature membrane exposure. In this review, we identified this complication in 11 out of 41 cases, accounting for 26% of the total cases. The highest incidence was observed in the Souidan et al. [21] study, with a rate of 90% (9 cases). One instance of bone graft loss was noted following early membrane exposure, but no cases of bone graft failure were reported in any of the early exposure cases (Table 7).
4. DISCUSSION
This study aimed to conduct a systematic review of the literature on the use of customized zirconia membranes for alveolar ridge augmentation. The review specifically focused on case series and case reports, as no randomized clinical studies, cohort studies, or long-term clinical studies were found in the literature. The included articles in this review demonstrated a consistent approach in terms of design and surgical procedures, with only minor variations among them.
All authors of the articles included in this review utilized cone beam computed tomography (CBCT) scans for designing custom membranes. They employed special software to convert DICOM formats into STL formats understood by 3D printing machines. The fabrication of all membranes was carried out using CAD/CAM technology.
Except for two surgical case reports [22], the customized membrane designs were non-porous. The porosity of barrier membranes remains a topic of significant controversy among authors [30-32]. The non-porous nature of the barrier membranes offers two crucial conditions for guided bone regeneration: the ability to maintain space and effective barrier capacity [32]. The barrier capacity aims to prevent unwanted cells from infiltrating the bone defect area. Pores in porous membranes can allow these cells to penetrate, reducing the predictability of bone volume formation [32]. On the other hand, non-porous membranes block the blood supply from the tissues surrounding the defect area and limit the regenerative potential of the periosteum [31, 32]. The periosteum houses mesenchymal cells capable of osteoblast differentiation [30]. Porous membranes offer the advantage of preventing bacterial invasion of the defect area if the membrane becomes exposed [20, 24]. However, the presence of holes in the zirconia membrane may increase the risk of fracture, either during the milling stage or the fixation stage, due to its inherent brittleness [25].
All the membranes in the reviewed articles were designed as a single piece, except in Joseph's case report [25], where the customized zirconia membrane was designed as two pieces that could be bonded together (Fig. 5). Joseph et al. [25] rationalized this design, stating that it eases the insertion of bone graft material under the membrane, ensuring a complete seal and eliminating gaps. Joseph et al. [25] also mentioned another rationale for this design, stating that the two-piece membrane design makes it easier to remove in cases where there is an undercut area hindering the device's removal. Furthermore, this design could potentially facilitate the creation of larger membranes suitable for accommodating large bone defects [25].
All authors noted the snug fit of the membrane against the host bone, leading to improved primary stability of the membranes and a reduced need for screws during fixation. This close fit not only prevents the loss of bone graft material and bacterial invasion when the membrane is exposed but also streamlines the process by eliminating the time and effort needed for conditioning, as compared to prefabricated membranes and meshes [20, 25, 26].
All authors employing non-porous membranes observed the development of a soft tissue layer, termed pseudoperiosteum, between the membrane and the newly formed bone [20, 21, 23-26]. Although the specific mechanism underlying the formation of this layer remains unclear, authors conducting histological analyses of this layer noted its absence of adverse effects or inflammatory responses, showcasing the excellent biocompatibility of zirconia [33]. An additional benefit of this layer is the ease of membrane removal [24, 25]. It is notable in all cases that the membranes lack integration and adhesion to soft and hard tissues, a phenomenon that remains unclear but aids in the straightforward removal of the membrane. This stands in contrast to other materials used in membrane production, which may necessitate significant time and effort for membrane removal [24].
Space maintenance is a crucial aspect of bone augmentation procedures, particularly in vertical bone defects [34-36]. Zirconia membranes have demonstrated effective space maintenance, which is attributed to their strength, which withstands soft tissue stress and prevents space collapse [20-26]. This phenomenon is often attributed to the thorough filling of the designated space with living bone, as emphasized by the majority of authors [20, 21, 24]. While hardness and toughness are crucial for space maintenance, zirconia exhibits brittleness, fragility, and inflexibility, rendering it intolerant to design or manufacturing errors and susceptible to breakage during milling or fixation [20, 22-24]. In a study conducted by Albash et al. [26], they opted to fabricate two identical membranes as a precaution. Mandeli et al. [24] highlighted the importance of careful screw tightening during fixation to prevent over-tightening and potential breakage.
In our review, it was noted that authors utilized various types of bone grafting materials, including autologous, allogeneic, and xenogeneic, except for alloplastic, and in some cases, no bone grafts were placed beneath the membranes. Heikal et al. [23], in their study comparing the use of zirconia membranes with and without grafts, highlighted a statistically significant difference in the volume and density of bone formation, favoring the group that received grafts [23]. The studies did not include follow-up periods exceeding 7 months, prompting consideration regarding the feasibility of employing bone grafts necessitating extended healing durations [20]. Malmstrom et al. [20], in their study involving three cases, reported a follow-up period of approximately 7 months, noting no adverse reactions to the presence of the membranes within the tissues [20]. Consequently, they proposed the potential use of zirconia membranes in conjunction with bone grafts requiring extended healing periods, such as alloplastic grafts [20].
Concerning premature membrane exposure, the majority of authors observed excellent soft tissue responses to zirconia membranes, with minimal instances of premature exposure reported during the follow-up period. In our review, the membrane exposure rate was found to be 26%, predominantly in the study by Souidan et al. (90% of cases) [21]. When excluding the Souidan et al. study from this review, the exposure rate decreased significantly to only 6.5%, which is notably lower than the exposure rates associated with prefabricated [37] or customized titanium meshes [38]. Souidan et al. [21] attributed the high rate of premature membrane exposure to factors such as design errors (suboptimal membrane design or excessive palatal extension), surgical errors (absence of periosteal sutures or palatally based incision line), or the specific location of the bone defect (anterior area of the maxilla characterized by strong muscle pull due to lip movement) [21].
CONCLUSION
While all studies included in this review were case series or case reports, customized zirconia membranes are highly effective in both localized and generalized bone augmentation procedures. These membranes exhibit high biocompatibility, superior space-maintenance capacity, excellent barrier capacity (non-porosity), ease of removal, and a close fit with the host bone.
Compared to custom or prefabricated titanium membranes, zirconia membranes display a relatively low rate of exposure. Their snug fit and excellent primary stability serve to safeguard the bone graft material from potential loss or bacterial contamination in case of membrane exposure. Nonetheless, caution is advised during usage due to their brittleness and vulnerability to fracture under high force, particularly during screw tightening.
We recommend conducting long-term randomized clinical trials to provide a more thorough evaluation of their efficacy. Additionally, we suggest comparing porous membranes with non-porous membranes in clinical trials, as well as exploring the use of alloplastic bone grafts beneath these membranes.
AUTHORS’ CONTRIBUTIONS
Z.A., A.K.: Study conception and design; Z.A., A.K., W.A.: Data collection; Z.A., A.K., W.A.: Analysis and interpretation of results; Z.A.: Draft manuscript.
LIST OF ABBREVIATIONS
| CBCT | = Cone Beam Computed Tomography |
| DICOM | = Digital Imaging and Communications in Medicine |
| CAD/CAM | = Computer-Aided Design and Manufacturing |
| JBI | = Joanna Briggs Institute |