All published articles of this journal are available on ScienceDirect.
The Effect of Dexamethasone Sodium Phosphate on Surgical Third Molar Extraction using Pain, Trismus, Oedema, and Quality of Life as Parameters: A Comparative Study
Abstract
Introduction
Lower third molar extractions are a common surgical procedure that can lead to post-operative complications such as trismus, discomfort, and swelling. One of the several corticosteroids frequently used to treat these issues is dexamethasone. This study aimed to assess the effects of submucosal and intramuscular injections of dexamethasone sodium phosphate on postoperative outcomes after surgical lower third molar extraction.
Materials and Methods
A total of 90 people were randomly allocated to one of three groups: submucosal dexamethasone (8 mg/2ml), intramuscular dexamethasone (8 mg/2ml), and a control group. Participants were evaluated for mouth opening, pain (Visual Analog Scale), oedema, and oral health-related quality of life (OHIP-14 Arabic) on the day of surgery and the third and seventh post-operative days.
Results
In comparison to the control group, the intramuscular dexamethasone groups showed noticeably improved results in terms of mouth opening, pain thresholds, and oedema measurements. On the seventh post-operative day, the intramuscular dexamethasone group demonstrated higher gains in mouth opening and reduced pain scores and oedema measurements.
Conclusion
Regardless of the method, administering dexamethasone effectively lowers post-operative problems after the lower third molar extraction, but an overall decrease in oral health and quality of life was observed. Submucosal delivery of dexamethasone can be a good alternative, even though intramuscular delivery may be somewhat more effective in some cases. These data support the use of dexamethasone as an effective adjuvant therapy in oral and maxillofacial surgery.
1. INTRODUCTION
The extraction of lower third molars is one of the most common surgical interventions conducted by oral and maxillofacial surgeons [1]. Despite its routine nature, this procedure frequently results in several post-operative complications, including pain, swelling, and trismus, primarily due to the inflammatory response induced by surgical trauma [2]. These complications can significantly impair a patient's quality of life (QOL), causing substantial discomfort and potentially leading to additional financial burdens from prolonged recovery periods and further treatments [3]. Given the high prevalence of these issues, there is a critical need for effective strategies to manage post-operative discomfort and enhance recovery [3].
Corticosteroids, first introduced in the 1950s with hydrocortisone, have been extensively used in dental practice as anti-inflammatory agents to mitigate post-operative inflammation [4]. These drugs work by inhibiting the synthesis and release of proinflammatory mediators, thereby reducing oedema and fluid transudation. Among them, dexamethasone sodium phosphate is particularly notable due to its potent anti-inflammatory properties and minimal mineralocorticoid effects [5]. Administered intramuscularly or submucosally, dexamethasone can significantly alleviate post-operative swelling, pain, facial oedema, and trismus by inhibiting leukocyte chemotaxis and stabilizing cellular membranes, enhancing patients' quality of life. However, there remains no consensus on the optimal administration route and regimen for its use [6].
Pain, primarily driven by inflammation from tissue damage, is a key concern after surgery, and while the Visual Analogue Scale (VAS) is commonly employed to assess patient pain levels, research suggests that dexamethasone has a stronger impact on reducing swelling and trismus than on pain itself [7]. Studies indicate that submucosal or intramuscular administration of dexamethasone can reduce facial oedema by minimizing fluid accumulation, typically measured at specific anatomical landmarks [8]. Trismus, characterized by limited mouth opening due to muscle and nerve immobilization, is also improved through dexamethasone use, as measured by interincisal distance [9]. Moreover, dexamethasone has been linked to decreased analgesic requirements post-surgery, reducing the need for NSAIDs, which can cause gastrointestinal side effects. When used in combination with NSAIDs, dexamethasone offers a synergistic effect, further minimizing post-operative discomfort [10]. Doses of 4 mg, delivered submucosally or intramuscularly, have been commonly employed to enhance post-operative recovery, improving patients' quality of life by alleviating symptoms that impact daily functioning, social interactions, and overall well-being [11].
Dexamethasone, a synthetic corticosteroid approxi- mately twenty-five times more potent than hydrocortisone, is known for its superior water solubility and clinical efficacy in managing post-operative complications following minor oral surgeries [12]. Its anti-inflammatory mechanism involves the inhibition of phospholipase A2, thereby reducing the production of key inflammatory mediators such as prostaglandins, leukotrienes, and thromboxanes. This action significantly mitigates the inflammatory response, which typically peaks two days post-surgery [13]. While intramuscular dexamethasone injections are widely practiced due to their rapid onset and sustained plasma levels, submucosal injections, though less studied, particularly in third molar surgery, offer a promising alternative. Delivering the drug near the surgical site, submucosal injections can effectively reduce localized inflammation and post-operative discomfort, presenting a safe, non-invasive, and cost-effective therapeutic option [14].
The use of corticosteroids is not without contra- indications, as various conditions can preclude their use, such as diabetes mellitus, peptic ulcers, tuberculosis, hypertension, glaucoma, and pregnancy [15]. These contraindications necessitate careful patient selection and dosage regulation to avoid adverse effects [16]. To address the gap in the literature regarding the most effective administration route for reducing post-operative compli- cations, the present study aims to compare the efficacy of dexamethasone sodium phosphate administered via two different approaches—intramuscular injection in the gluteal muscle and submucosal injection in the oral vestibule buccal to the third molar area—on postoperative outcomes following the surgical extraction of lower third molars. This study will specifically evaluate the effects of these administration routes on pain, swelling, trismus, and Oral Health-related Quality of Life (OHRQoL) using the Oral Health Impact Profile (OHIP-14) Arabic version.
By comparing these two administration routes, this study seeks to provide evidence-based guidance on the most effective method to mitigate post-operative complications and enhance recovery for patients under- going lower third molar extractions. The goal is to determine whether a single dose of dexamethasone administered submucosally can significantly improve post-operative QOL measures comparable to intramuscular injection. This research can inform clinical practice, improve patient outcomes, and ultimately contribute to optimising post-operative care in oral and maxillofacial surgery [17].
2. MATERIALS AND METHODS
2.1. Targeted Population
Our targeted population is the dental patients visiting dental clinics at the dental college, Taibah University, Saudi Arabia. Ethical approval was obtained from Taibah University, College of Dentistry Research Ethics Committee (TUCDREC/21O223/MHAliohani).
2.2. Sample Size
The total number of participants was 90 participants. The sample size based on the number of patients in the previous year was 120 had been treated for surgical lower third molar extraction and the time period for this study is 1 year; the sample size with the confidence level is 95%, marginal error of 5, and population proportion of 50 should be 90 divided into three equal groups. So it should be 90 participants in total and 30 in each group.
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
A) Patients attending dental clinics at dental college, Taibah University, for all surgical lower third molar extractions (irrespective of the state of surgical extraction based on the Winters or Pell and Gregory classification).
B) Adult female or male patients ≥16 years old.
C) Healthy patients with no severe medical problems and, according to the American Society of Anesthesio- logists (ASA), must be ASA I or ASA II.
D) Patients who can consent for themselves with or without an interpreter.
2.3.2. Exclusion Criteria
A) Young patient <16 years old.
B) Patient with severe medical or psychological problems.
C) Patient who is unable to consent for him/herself.
D) Non-surgical extraction
Therefore, any patient who has fulfilled the inclusion criteria and is willing to consent after being assured that participation in this study is completely voluntary, and if they refuse to participate at any time, will have no consequences for them. They will be asked to participate in this research project and will be asked to fill out the OHIP-14 in Arabic before the start of their treatment and one week after completion of surgical extraction treatment, either at their follow-up visits or over the phone.
2.4. Distributing Criteria
They were randomly distributed into 1 of 3 groups: submucosal 8 mg dexamethasone sodium phosphate (30 participants), dexamethasone sodium phosphate intra- muscularly 8 mg (30 participants), and a control group that received no steroid (30 participants). Two participants started the treatment but lost contact and were excluded from the study.
2.5. Intervention Procedure
In the study, both submucosal and intramuscular injections were administered using disposable 3 mL syringes equipped with 23-gauge, 1-inch needles. For the submucosal injections, the anatomical reference point was located buccally to the lower third molar area in the buccal vestibule, approximately 20 mm from the gingival margin of the adjacent tooth. The volume of dexa- methasone sodium phosphate administered for both techniques was 8 mg in a 2 mL solution. Intramuscular injections were targeted to the upper outer quadrant of the gluteal region.
2.6. Data Collection
The participants were assessed on the day of the surgery and the third day post-operatively, then the seventh day at their follow-up visits for their pain, swelling, and trismus. The exception for the assessment time was the modified translated questionnaire, which was used to assess the patient's perception regarding different quality of life dimensions (OHIP-14). Participants were asked twice, first before the start of the treatments and second on the seventh day post-op, to assess their OHRQoL in the past 7 days. A comprehensive medical history was obtained to confirm the necessity for third-party intervention, and an oral examination, including an orthopantomogram radiograph, was conducted to confirm the need for third molar extraction.
2.7. Sampling Approach
A random selection was made for each participant to be included in one of the groups after an explanation of the procedures and the written consent form were taken from each participant in accordance with the Declaration of Helsinki. All the participants were given local anes- thesia (lidocaine 2% with epinephrine 1:200,000) at the surgical site. Third molar extraction was done surgically after the inferior alveolar nerve was given local anesthesia and the buccal fold was terminally infiltrated. For the postoperative management, post-operative instructions were given orally and written, and a nonsteroidal anti-inflammatory drug (Paracetamol 500 mg, 2 tablets every 6 hours for 3 days) and amoxicillin/clavulanic acid, 1 gram tablet every 12 hours for 5 days, were prescribed.
2.9. Variables Collection
2.9.1. Mouth Opening (trismus)
The patient was evaluated before the start of the treatment by measuring their mouth opening from the incisal edge of the upper central incisor to the lower opposing lower central incisor before the start of the treatment, then at the third day post-operatively, then the seventh day post-operatively.
2.9.2. The Size of Post-operative Oedema
The size of post-operative oedema was determined before the procedure (baseline level), after 3 days post-operatively, and finally on the seventh-day post-op. The distance between the tip of the chin and the lower part of the auricle lobe was measured, and the oedema coefficient (Ec) was calculated using the modified formula of Carrillo, Ec ¼ post-operative distance pre-operative distance 100 Statistical analysis of oedema coefficient differences between the groups were performed using the non-parametric Wilcoxon rank test [18].
2.9.3. Pain
Visual Analog Scale (VAS) out of 10 was used, asking the patient if there is a pin, how would they rate it from 0 (there is no pain) to 10 (the worst pain they have ever felt), where is 0 “no pain,” 1 to 3 “mild pain,” 4 to 6 “moderate pain,” 7 to 9 “severe pain” and 10 very severe pain. The VAS was recorded on the day of the procedure (pre-op), the third day, and the seventh day.
2.9.4. Oral Health-related Quality of Life (OHRQoL)
Patients were asked to fill the OHRQ0L using an oral health profile (OHIP-14) in Arabic questionnaire before the start of their treatment and then at the seventh day of treatment (follow-up visit) asking them how to rate the OHRQoL in the past week.
OHIP-14 in Arabic was chosen as it was the most frequent tool for measuring OHRQoL. In addition, OHIP-14 in Arabic has been examined in many studies [19]. The general question was as follows: “Is there a negative impact of your oral health on your overall QoL?” the answer was like the OHIP-14, a five-point scale ranging from 0 (never), 1 (hardly ever), 2 (occasionally), 3 (fairly often), 4 (very frequently).
2.10. Data Analysis
The data is entered, organized, tabulated, and analyzed using the standard computer program Statistical Package of Social Sciences (SPSS) version 24.0. Qualitative data is expressed as numbers and percentages (No & %). The chi-square (x2) test assesses the association between two or more qualitative variables. Quantitative data is presented as mean and standard deviation (mean+/-SD). Student t-test is used for comparing two quantitative normally distributed variables, and ANOVA test for comparing more than two quantitative customarily distributed variables with the significant Level set at P-value < 0.05.
3. RESULTS
Table 1 is the descriptive table with the mean age of participants at 29 years (SD 7.203, range 17-52 years). Pre-operative mouth opening averaged 39.76 mm (SD 4.572, range 27-47 mm), reducing to 33.76 mm (SD 4.909, range 21-42 mm) on day 3 and improving to 35.76 mm (SD 4.909, range 23-44 mm) by day 7. Pain scores (VAS) declined from a pre-operative mean of 4.32 (SD 1.779, range 1-9) to 2.19 (SD 1.027, range 1-5) on day 3 and 1.08 (SD 0.902, range 0-4) by day 7. Oedema increased from a pre-operative mean of 148.26 mm (SD 33.171, range 100-228 mm) to 182.86 mm (SD 41.797, range 120-300 mm) on day 3, then decreased to 170 mm (SD 38, range 110-280 mm) by day 7. These trends indicate post-operative recovery with reduced pain, improved mouth opening, and decreased oedema by the seventh day.
Table 2 examined the impact of various dexa- methasone delivery routes on surgical outcomes by calculating the mean and p-value derived from one-way ANOVA. Initially, the average mouth opening was 39.5 mm, decreasing by day 3 across all groups. The control group had the lowest average mouth openness (31.4 mm), while dexamethasone-treated groups showed improve- ments (p=0.002). By day 7, all groups showed improve- ment, with the intramucosal dexamethasone group exhibiting the most significant recovery (37.5 mm) (p=0.002). Pain scores decreased significantly by day 3, with dexamethasone-treated groups showing lower scores than the control (1.4 for intramuscular and 2.1 for submucosal) (p=0.000). By day 7, pain scores decreased, with the dexamethasone groups having the lowest scores (0.5 for intramuscular and 1.0 for submucosal) (p=0.000). Oedema increased by day 3 for all groups, with dexamethasone-treated groups showing lesser increments (p=0.178). By day 7, oedema decreased, with dexamethasone-treated groups having the lowest measurements (p=0.228). Overall, dexamethasone, particularly intramuscular administration, effectively reduced pain, and improved mouth opening and oedema, suggesting its importance in post-surgical healing.
| Variables | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|
| Age | 17 | 52 | 29.00 | 7.203 |
| Mouth opening pre-operative | 27 | 47 | 39.76 | 4.572 |
| Mouth opening post-op 3 | 21 | 42 | 33.76 | 4.909 |
| Mouth opening post-7 | 23 | 44 | 35.76 | 4.909 |
| Pain VAS pre-operative | 1 | 9 | 5.27 | 1.779 |
| Pain VAS 3rd day | 1 | 5 | 2.19 | 1.027 |
| Pain VAS 7th day | 0 | 4 | 1.08 | .902 |
| Oedema measurement pre in mm | 100 | 228 | 148.26 | 33.171 |
| Oedema measurement post-op 3rd day | 120 | 300 | 182.86 | 41.797 |
| Oedema measurement post-op 7th day | 110 | 280 | 170 | 38 |
| Variable | Control Group | Submucosal Dexamethasone | Intramuscular Dexamethasone | p-value |
|---|---|---|---|---|
| Mouth opening(mm) | ||||
| Pre-op | 39.4 | 39.9 | 39.5 | 0.842 |
| Post-op day 3 | 31.4 | 33.9 | 35.5 | 0.002 |
| Post-op day 7 | 33.4 | 35.9 | 37.5 | 0.002 |
| Pain (VAS) | ||||
| Pre-op | 5.3 | 5.1 | 5.1 | 0.990 |
| Post-op day 3 | 2.9 | 2.1 | 1.4 | 0.000 |
| Post-op day 7 | 1.6 | 1 | 0.5 | 0.000 |
| Oedema measurement(mm) | ||||
| Pre-op | 148.8 | 148.8 | 145.7 | 0.980 |
| Post-op day 3 | 193.5 | 181.5 | 171.7 | 0.178 |
| Post-op day 7 | 180.3 | 168.4 | 162 | 0.228 |
Table 3 presented pre-operative and post-operative issues based on the OHIP-14 scale and the p-value was calculated using a paired-sample t-test. Post-surgery, difficulty pronouncing words increased significantly, with “never” declining to 34.4% and “very often” rising to 15.6% (p=0.000). Taste sense deterioration also increased significantly, with “never” decreasing to 37.8% and “very often” rising to 12.2% (p=0.000). Painful mouth aches and eating discomfort showed significant increases in “very often” occurrences (p=0.000). Self-consciousness increased, with “never” falling to 6.7% and “very often” rising to 44.4% (p=0.000). Notable rises were observed in tenseness, diet dissatisfaction, meal interruptions, trouble relaxing, embarrassment, irritability, incapacity to function, social difficulties, and diminished life fulfillment post-surgery, indicating substantial functional, physical, psychological, and social limitations and handicap difficulties during the post-operative phase.
The study's participants showed notable post-operative changes in their impact profile related to oral health. The average ratings for functional restrictions, like difficulty pronouncing words and a diminished sense of taste, increased significantly (p=0.000), from 0.63 to 1.54 and 0.46 to 1.42, respectively. Physical pain, such as excruciating aching and discomfort after eating, also rose (p=0.000) from 2.51 to 3.09 and 2.49 to 3.09, respectively. Significant increases were observed in psychological discomfort and impairments, including self-consciousness and tension, between pre-operative and post-operative measures (p=0.000). These findings showed that after the intervention, there was a discernible deterioration in oral health and well-being (Table 4).
|
Variables (OHIP-14) |
Frequency (n=90) |
Percentage (%) |
Frequency (n=90) |
Percentage (%) |
Confidence interval (95%) p-value |
|
|---|---|---|---|---|---|---|
| Pre-operative | Post-operative | |||||
| Functional limitation: Had trouble pronouncing any words | -1.137 - -.685 | 0.000 | ||||
| Never | 62 | 68.9 | 31 | 34.4 | ||
| Hardly ever | 10 | 11. 1 | 18 | 20.0 | ||
| Occasionally | 9 | 10.0 | 16 | 17.8 | ||
| Fairly often | 7 | 7.8 | 11 | 12.2 | ||
| Very often | 2 | 2.2 | 14 | 15.6 | ||
| Functional limitation: Felt sense of taste has worsened | -1.203 - -.731 | 0.000 | ||||
| Never | 69 | 76.7 | 34 | 37.8 | ||
| Hardly ever | 8 | 8.9 | 18 | 20.0 | ||
| Occasionally | 8 | 8.9 | 15 | 16.7 | ||
| Fairly often | 3 | 3.3 | 12 | 13.3 | ||
| Very often | 2 | 2.2 | 11 | 12.2 | ||
| Physical pain: Painful aching in the mouth | -.761 - -.395 | 0.000 | ||||
| Never | 13 | 14.4 | 7 | 7.8 | ||
| Hardly ever | 8 | 8.9 | 5 | 5.6 | ||
| Occasionally | 15 | 16.7 | 10 | 11. 1 | ||
| Fairly often | 28 | 31. 1 | 19 | 21. 1 | ||
| Very often | 26 | 28.9 | 49 | 54.4 | ||
| Physical pain: found it uncomfortable to eat any food | -.785 - -.415 | 0.000 | ||||
| Never | 9 | 10.0 | 6 | 6.7 | ||
| Hardly ever | 15 | 16.7 | 9 | 10.0 | ||
| Occasionally | 15 | 16.7 | 7 | 7.8 | ||
| Fairly often | 25 | 27.8 | 17 | 18.9 | ||
| Very often | 26 | 28.9 | 51 | 56.7 | ||
| Psychological discomfort: been self-conscious | -.833 - -.434 | 0.000 | ||||
| Never | 14 | 15.6 | 6 | 6.7 | ||
| Hardly ever | 16 | 17.8 | 11 | 12.2 | ||
| Occasionally | 19 | 21. 1 | 14 | 15.6 | ||
| Fairly often | 19 | 21. 1 | 19 | 21. 1 | ||
| Very often | 22 | 24.4 | 40 | 44.4 | ||
| Psychological discomfort: felt tense | -.831 - -.436 | 0.000 | ||||
| Never | 20 | 22.2 | 9 | 10.0 | ||
| Hardly ever | 14 | 15.6 | 12 | 13.3 | ||
| Occasionally | 20 | 22.2 | 16 | 17.8 | ||
| Fairly often | 14 | 15.6 | 15 | 16.7 | ||
| Very often | 22 | 24.4 | 38 | 42.2 | ||
| Physical disability: felt diet has been unsatisfactory | -.831 - -.436 | 0.000 | ||||
| Never | 21 | 23.3 | 10 | 11. 1 | ||
| Hardly ever | 11 | 12.2 | 10 | 11. 1 | ||
| Occasionally | 22 | 24.2 | 17 | 18.9 | ||
| Fairly often | 13 | 14.4 | 13 | 14.4 | ||
| Very often | 23 | 25.6 | 40 | 44.4 | ||
| Physical disability: had to interrupt meals | -.943 - -.523 | 0.000 | ||||
| Never | 34 | 37.8 | 16 | 17.8 | ||
| Hardly ever | 16 | 17.8 | 19 | 21. 1 | ||
| Occasionally | 20 | 22.2 | 16 | 17.8 | ||
| Fairly often | 6 | 6.7 | 11 | 12.2 | ||
| Very often | 14 | 15.6 | 28 | 31. 1 | ||
| Psychological disability: found it difficult to relax | -.969 - -.542 | 0.000 | ||||
| Never | 38 | 42.2 | 19 | 21. 1 | ||
| Hardly ever | 16 | 17.8 | 14 | 15.6 | ||
| Occasionally | 10 | 11. 1 | 16 | 17.8 | ||
| Fairly often | 16 | 17.8 | 17 | 18.9 | ||
| Very often | 10 | 11. 1 | 24 | 26.6 | ||
| Psychological disability: been a bit embarrassed | -.950 - -.516 | 0.000 | ||||
| Never | 34 | 37.8 | 16 | 17.8 | ||
| Hardly ever | 10 | 11. 1 | 12 | 13.3 | ||
| Occasionally | 23 | 25.6 | 18 | 20.0 | ||
| Fairly often | 6 | 6.7 | 16 | 17.8 | ||
| Very often | 17 | 18.9 | 28 | 31. 1 | ||
| Social disability: been a bit irritable | -1. 182 - -.707 | 0.000 | ||||
| Never | 64 | 71. 1 | 32 | 35.6 | ||
| Hardly ever | 9 | 10.0 | 14 | 15.6 | ||
| Occasionally | 9 | 10.0 | 16 | 17.8 | ||
| Fairly often | 4 | 4.4 | 18 | 20.0 | ||
| Very often | 4 | 4.4 | 10 | 11. 1 | ||
| Social disability: had difficulty doing usual jobs | -1. 176 - -.713 | 0.000 | ||||
| Never | 43 | 47.8 | 20 | 22.2 | ||
| Hardly ever | 14 | 15.6 | 14 | 15.6 | ||
| Occasionally | 24 | 26.7 | 23 | 25.6 | ||
| Fairly often | 7 | 7.8 | 16 | 17.8 | ||
| Very often | 2 | 2.2 | 17 | 18.9 | ||
| Handicap: felt life less satisfying | -.878 - -.477 | 0.000 | ||||
| Never | 28 | 31. 1 | 14 | 15.6 | ||
| Hardly ever | 21 | 23.3 | 17 | 18.9 | ||
| Occasionally | 11 | 12.2 | 14 | 15.6 | ||
| Fairly often | 13 | 14.4 | 14 | 15.6 | ||
| Very often | 17 | 18.9 | 31 | 34.4 | ||
| Handicap: been unable to function | -1. 188 - -.723 | 0.000 | ||||
| Never | 42 | 46.7 | 21 | 23.3 | ||
| Hardly ever | 28 | 31. 1 | 20 | 22.2 | ||
| Occasionally | 12 | 13.3 | 19 | 21. 1 | ||
| Fairly often | 6 | 6.7 | 14 | 15.6 | ||
| Very often | 2 | 2.2 | 16 | 17.8 | ||
|
Variables (OHIP-14) |
Pre-operative (mean) |
Post-operative (mean) |
p-value |
|---|---|---|---|
| Functional limitation: Had trouble pronouncing any words | 0.63 | 1.54 | 0.000 |
| Functional limitation: Felt sense of taste has worsened | 0.46 | 1.42 | 0.000 |
| Physical pain: Painful aching in the mouth | 2.51 | 3.09 | 0.000 |
| Physical pain: found it uncomfortable to eat any food | 2.49 | 3.09 | 0.000 |
| Psychological discomfort: been self-conscious | 2.21 | 2.84 | 0.000 |
| Psychological discomfort: felt tense | 2.04 | 2.68 | 0.000 |
| Physical disability: felt diet has been unsatisfactory | 2.07 | 2.70 | 0.000 |
| Physical disability: had to interrupt meals | 1.44 | 2.18 | 0.000 |
| Psychological disability: found it difficult to relax | 1.40 | 2.16 | 0.000 |
| Psychological disability: been a bit embarrassed | 1.58 | 2.31 | 0.000 |
| Social disability: been a bit irritable | 0.61 | 1.56 | 0.000 |
| Social disability: had difficulty doing usual jobs | 1.21 | 1.96 | 0.000 |
| Handicap: felt life less satisfying | 1.67 | 2.34 | 0.000 |
| Handicap: been unable to function | 0.87 | 1.82 | 0.000 |
Before the surgical intervention, the mouth-opening measurements of the three study groups were comparable in Fig. (1). Before surgery, the three study groups had similar baseline mouth-opening measurements (p=0.842). On the third post-operative day, all groups experienced a reduction in mouth opening, with the dexamethasone-treated groups showing less decrease than the control (p=0.002). By the seventh post-operative day, all groups showed improvement, with the submucosal dexa- methasone group nearly reaching pre-operative levels (p=0.002). The intramuscular dexamethasone group showed slightly more improvement than the submucosal group.
In contrast, the control group's improvement was less pronounced. Overall, submucosal or intramuscular dexa- methasone administration effectively mitigated post-operative mouth opening reduction, facilitating quicker recovery than the control group. By day 7, mouth-opening values in the intramucosal dexamethasone group had returned to pre-operative levels, indicating the most favorable outcome.
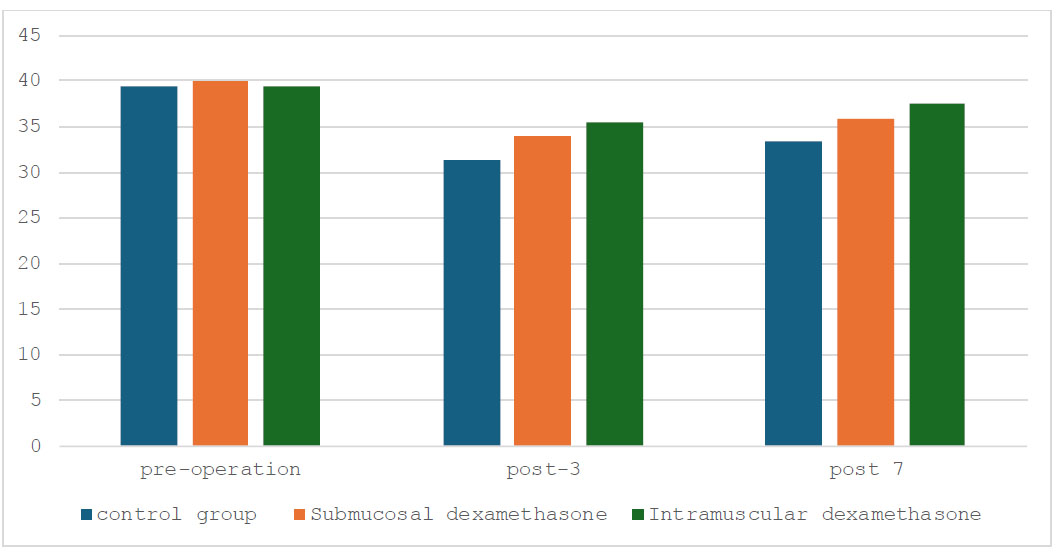
Mouth opening on pre-operative day, post-operative days 3 and 7.
Fig. (2) interprets the pain visual analog scale on pre-operative day and post-operative days 3 and 7. On the day before surgery, all groups reported similar degrees of pain, around 5 (p=0.990). All groups had decreased pain on post-operative (p=0.000) day 3 compared to pre-operative pain, and the intramuscular group had slightly lower average pain scores than the submucosal dexa- methasone group. Also, the pain persisted and subsided by the seventh day following surgery, and the intramucosal dexamethasone group demonstrated even more improve- ment (p=0.000). The control group did not decrease; however, it did stay higher than the two treatment groups. In conclusion, this figure showed that administering dexamethasone, particularly intramuscularly, reduced post-operative pain.
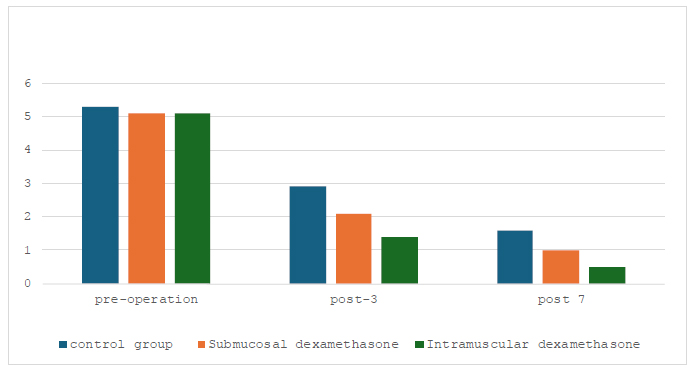
Pain visual analog scale on the pre-operative day, post-operative days 3 and 7.
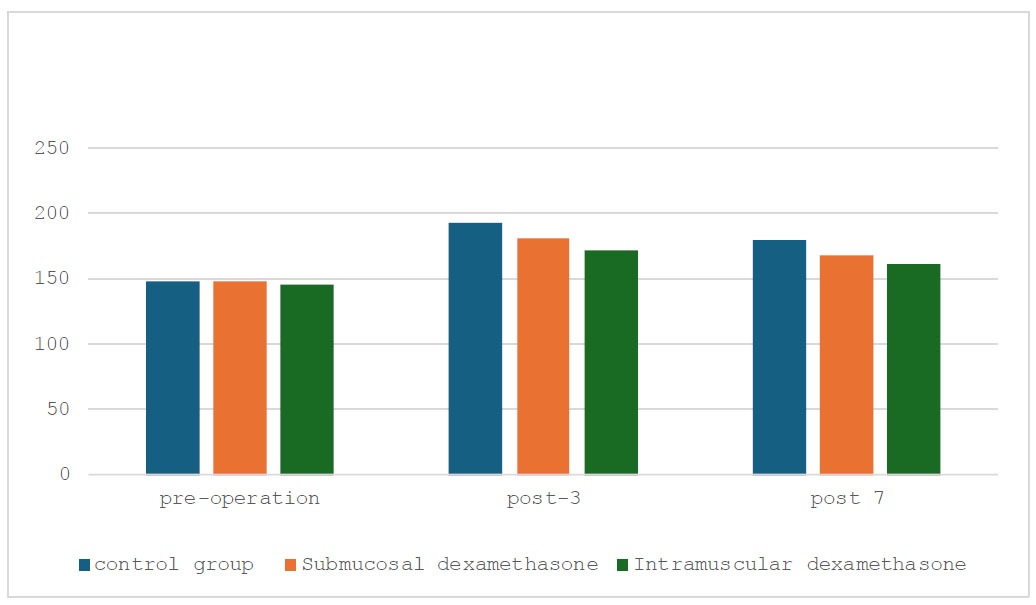
Oedema measurement on a pre-operative day, post-operative days 3 and 7.
Fig. (3) analyzes the oedema measurement on pre-operative day, post-operative days 3 and 7. At first, the oedema readings in all groups were comparable, with values slightly under 200mm. pre-operative oedema levels were similar in the control, submucosal, and intramuscular dexamethasone groups (p=0.980). Both dexamethasone treatment groups showed reduced oedema by the third post-operative day compared to the control group. Oedema readings in the intramucosal dexamethasone group were approximately 172mm, indicating a more notable reduction (p=0.178).
All groups' oedema readings kept declining on post-operative day 7. At about 162mm, the intramucosal dexamethasone group retained the least amount of oedema (p=0.228). In conclusion, this figure illustrated how various interventions affected the amount of oedema after surgery. Notably, when compared to no treatment (control) within seven days post-surgery, intramucosal infusion of dexamethasone seemed more successful in reducing oedema than intramuscular administration.
The distribution of pre-operative pain (as determined by the Visual Analog Scale, or VAS) by gender is shown in Fig. (4). The pain scale went from 0 to 10 for both genders. The highest numbers were seen in females with pain scores of 4 and 5, which each reached counts of roughly 13 and 10 people, respectively. Less frequently, scores 2, 3, 4, 7, 8, and 9 displayed lower counts compared to the other pain levels. Likewise, the most common pain scores among men were 5 and 6, with counts averaging about 10 and 8, respectively. While there was variation in the other pain scores, scores 2, 3, 4, 7, 8, and 9 had lower counts than scores 5 and 6. Overall, the data indicated that females experienced more pain.
The distribution of pain levels (VAS scores) by gender on the third post-operative day is shown in Fig. (4). It revealed that, among women, the most prevalent pain level was 2, as reported by more than thirty people. The next most common pain level was level 1, followed in decreasing order by levels 4, 3, and 5. In comparison, the distribution of pain levels was more even among males; approximately ten people reported experiencing pain at levels 1, 2, and 4, although levels 3 and 5 were less common. In general, women seemed to experience more pain than men did.
The distribution of pain levels (VAS scores) on the seventh post-operative day, broken down by gender, is shown in Fig. (4). It was found that, among females, over twenty-five claimed that the most common pain level was 1. Pain levels 0 and 2, involving roughly 20 and 15 people, respectively, came next. Levels 3 and 4 of pain were notably less prevalent. In the case of men, the distribution was more evenly distributed, with roughly 10 people reporting pain levels 0 and 2, and about 15 people reporting pain levels 1, which was the most common. Fewer people reported having pain levels 3 and 4. By the seventh day, higher pain levels were generally reduced in both genders, with females reporting lower pain levels more frequently than males.
4. DISCUSSION
The purpose of the current study was to compare the effects of submucosal injection in the oral vestibule buccal to the third molar area and intramuscular injection in the gluteal muscle on post-operative outcomes after lower third molar surgery. Pain, oedema, trismus, and OHRQoL were the primary outcomes that were assessed. Compared to the control group, the study's findings show that dexamethasone administration—regardless of the method—successfully lowered post-operative problems. Compared to the control group, the intramuscular and submucosal dexamethasone groups showed noticeably improved results regarding mouth opening, pain thresholds, and oedema measurements.
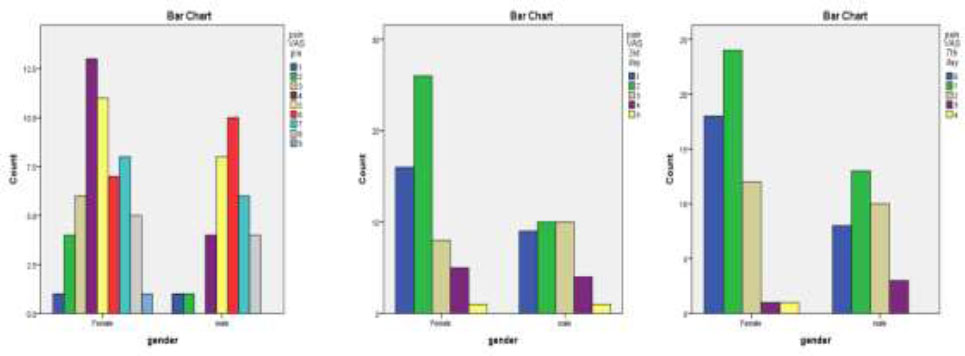
Gender differences in pain levels on the pre-third, and seventh-day post-operation.
Pain management is a crucial part of post-operative care since it influences the comfort and recovery of the patient. The findings indicate that, compared to the control group, dexamethasone delivered submucosally and intramuscularly significantly reduced pain, particularly on the third and seventh post-operative days. The intra- muscular group may have a more robust and longer-lasting analgesic impact because they had the lowest pain scores. Swelling is a common post-operative problem that can be highly uncomfortable and impair a patient's ability to eat and speak. According to the study, dexamethasone given via both routes successfully decreased post-operative edema by the seventh day. On the third post-operative day, however, the intramucosal group had a marginally more significant reduction in oedema than the submuscular group. In comparison to the control group, our data demonstrated a considerable improvement in mouth opening for both dexamethasone groups. On the seventh post-operative day, the intramuscular group showed a notable improvement in mouth opening. This could be because the drug's systemic distribution reduces inflammation more efficiently over time.
These results are in line with earlier research that documented the usefulness of dexamethasone in the treatment of post-operative complications following the excision of a third molar. A comprehensive review and meta-analysis by Markiewicz et al. indicated that administering corticosteroids, particularly dexamethasone, was related to reduced edema, trismus, and pain after third molar surgery [20]. In a similar vein, research by Boonsiriseth et al. and Gopinath et al. showed that trismus and post-operative oedema could be effectively reduced with a single 4 mg dosage of dexamethasone given submucosally or intramuscularly [21, 22]. In this study, Klongnoi et al. examined the effects on post-operative outcomes following lower-impacted third molar surgery with pre-operative intramuscular dexamethasone injection at a single dose of 8 mg. Results revealed no discernible change in trismus between the dexamethasone and control groups. They decreased oedema and pain on the second and seventh post-operative days [23]. However, according to Priyanga et al.'s study, oral health-related quality of life after lower third molar extraction was improved by dexamethasone administration of 8 mg, regardless of the route, effectively reducing pain, swelling, and trismus. In specific measures, intramuscular dexamethasone showed slightly better results [24].
The current study's results indicate that intramuscular dexamethasone may be somewhat more beneficial than the submucosal route in improving specific outcomes when comparing the two administration routes. On the seventh post-operative day, the intramuscular group showed better improvements in mouth opening and reported less pain than the submucosal group. These results are consistent with those of Boonsiriseth et al. and Priyanga et al., who discovered that dexamethasone, whether injected intramuscularly or submucosally, successfully decreased discomfort, edema, and trismus and enhanced the quality of life linked to oral health after the extraction of the lower third molar [21, 24]. A study by Majid and Mahmood discovered that intramuscular dexamethasone had somewhat improved outcomes in various metrics [8]. Its pharmacokinetic characteristics could be the reason for intramuscular dexamethasone's better performance. When administered intramuscularly, medicine can enter the systemic circulation more quickly and sustainably, resulting in higher and more stable plasma concentrations [25]. Subcutaneous injection, on the other hand, can cause a more confined and possibly irregular dispersion of the medication, which might restrict its overall efficacy. It is crucial to remember that, in contrast to the control group, the intramucosal group in this study showed considerable improvements in post-operative outcomes. This implies that the administration of dexamethasone submucosally can also be a realistic and successful option, especially in circumstances where the patient may not find intramuscular injection to be feasible or desirable [26].
When OHRQoL was measured using the OHIP-14 questionnaire, the results indicated that the dexa- methasone groups significantly outperformed the control group, but we see a significant decline in overall oral health quality. In our findings, it is important to acknowledge that this deterioration may primarily result from the surgical intervention itself rather than the effects of dexamethasone. The nature of third molar extraction can inherently lead to discomfort, changes in functionality, and psychological distress, which could impact patients' perceptions of their oral health. Dexamethasone, as indicated by our results, effectively mitigates pain, swelling, and trismus, suggesting that while overall OHRQoL may decline due to surgical trauma, the treatment remains beneficial in alleviating specific post-operative complications. Thus, it is crucial to differentiate between the impacts of the surgery and the treatment in interpreting the outcomes. Majid and Mahmood's study shows that dexamethasone is given submucosally or intramuscularly to enhance oral health-related quality of life after lower third molar extraction [8]. Al-Sharaee et al. investigated the effects of dexamethasone and Traumeel S on oral health-related quality of life (OHRQoL) following lower third molar extraction. Comparing dexamethasone to Traumeel S, the results demonstrated a considerable improvement in OHRQoL, particularly in psychological disability and discomfort. This implies that after lower third molar extraction, dexamethasone might be a more useful adjunct medication for controlling post-operative problems and improving patient outcomes [27].
The current study's strengths include its complete assessment of post-operative complications and OHRQoL, and its use of validated outcome measures. Furthermore, the study population was precisely characterized, and the exclusion criteria were unambiguously stated, which improved the findings' internal validity.
The study does, however, have certain limitations. First, despite the significant findings of this study, one notable limitation is the relatively small sample size of 90 participants. This may limit the ability to detect subtle differences between the submucosal and intramuscular dexamethasone groups. Future research with larger sample sizes would be essential to validate these findings and provide a more comprehensive understanding of the comparative effectiveness of these delivery methods. Second, whereas long-term results beyond the 7-day follow-up may be crucial to comprehend the long-term consequences of dexamethasone administration, they were not evaluated in this investigation. Lastly, future research should consider the possible negative consequences of using dexamethasone, which were not assessed in this study.
The study advises the administration of dexa- methasone, either submucosally or intramuscularly, as an efficient adjunct therapy for managing post-operative complications after third molar extraction. This is because dexamethasone significantly lowers pain, swelling, and trismus. In specific measures, intramuscular dexa- methasone administration yields slightly better results. Future studies should look at the long-term effects and possible side effects of using dexamethasone. Larger-scale studies with longer follow-up times could also be required to clarify the relative effectiveness of the two delivery modalities.
CONCLUSION
The current study concludes that dexamethasone administration, by any method, helps lower post-operative problems and enhance the oral health-related quality of life after lower third molar extraction surgery. Intra- mucosal delivery of dexamethasone can be a good alternative, even though submuscular delivery may be somewhat more effective in some cases. Statistical analysis reveals notable improvements in pain manage- ment, reduction of swelling, and enhancement of mouth opening, particularly with intramuscular delivery, but overall decrease in oral health and quality of life observed. These findings add to the growing body of evidence supporting dexamethasone as an effective adjuvant therapy in oral and maxillofacial surgery.
AUTHOR'S CONTRIBUTION
The author confirms sole responsibility for the following: study conception and design, data collection, analysis andinterpretation of results, and manuscript preparation.
LIST OF ABBREVIATIONS
| QoL | = Quality of life |
| VAS | = Visual Analogue Scale |
| OHRQoL | = Oral Health-related Quality of Life |
| OHIP-14 | = Oral Health Impact Profile |
| ASA | = Anesthesiologists |
| SPSS | = Statistical Package of Social Sciences |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval was obtained from Taibah University, Saudi Arabia, College of Dentistry Research Ethics Committee (TUCDREC/21O223/MHAliohani).
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available at: https://shorturl.at/P0kbW.


