All published articles of this journal are available on ScienceDirect.
Comparative Assessment of the Shear Bond Strength of Green Synthetized Titanium Dioxide, Hydroxyapatite and Chitosan Nanoparticles Integrated in Orthodontic Adhesive and Evaluation of Antibacterial Ability, an In-vitro and Laboratory Study
Abstract
Background
Recently, nanoparticles have raised interest due to their antibacterial characteristics when added to composite adhesives, as the impact of the addition of nanoparticles on shear bond strength is not widely investigated.
Objective
The in Vitro and laboratory Study assessed the antibacterial ability and the shear bond strength (SBS) of greenly synthesized titanium dioxide nanoparticles bonded with orthodontic adhesive against those bonded to chitosan and hydroxyapatite.
Methods
The green titanium dioxide nanoparticles were prepared from Salvadora persica. Titanium dioxide nanoparticles were recorded via Fourier transform infrared spectroscopy analysis, the crystal appearance and morphology of titanium dioxide nanoparticles were investigated by UV–Visible reflectance spectrum, and the surface morphology was visualized using electron microscopy, and determination of titanium dioxide nanoparticles homogeneity and elemental distribution was achieved by energy dispersive X-ray spectrometry. An ex vivo study was performed using forty healthy extracted human premolars and randomly partitioned into four groups. The first group included brackets bonded with orthodontic adhesive (what brand, city, country) without nanoparticles; the second, third, and fourth groups contained titanium dioxide nanoparticles, chitosan nanoparticles alone, and chitosan with hydroxyapatite nanoparticles, respectively. SBS and the adhesive remnant index (ARI) scores were recorded. Measurement of SBS (MPa) of the different groups’ samples was performed 24 hours after preparation using a universal testing machine, and the adhesive remnant index was defined using a light stereomicroscope (Nikon SM2-10, Japan) at ×10 magnification. The prepared titanium dioxide nanoparticles were assessed for their antibacterial activity versus pathogenic Gram positive and negative bacterial species through disc diffusion assay. The SBS data were analyzed using debonding force, while the antibacterial assays were analyzed using SPSS 21 software via one-way ANOVA.
Results
The characteristic analysis provided the elemental structure of the prepared titanium dioxide nanoparticles in addition to metal crystallization, textural features, and functional bonds. Based on the extracted data, the control group revealed higher mean SBS (8.54 ± 0.76) in parallel to the three groups containing nanoparticles incorporated composites. The group comprising titanium dioxide nanoparticles exhibited the nearest value to the control group (8.38 ± 0.78) and higher to that of the other two groups, chitosan and chitosan and hydroxyapatite, (7.92±0.86) and (7.48 ± 0.46) respectively. However, according to the One Way ANOVA test, the SBS values were not significantly different across all four studied groups (p= 0.379). The ARI score of 1 was the most prevalent among the four groups as determined in 18 teeth at 45%, with no significant difference between bracket groups (p=0.171). The minimum bactericidal concentrations of 5 g/mL were 1x103 and 1x105 against Escherichia coli O25: K11 and Staphylococcus intermedius.
Conclusion
The results of our study, as well as the ex-vivo and in-vitro findings, verified that incorporating titanium dioxide nanoparticles into an orthodontic adhesive enhanced its antibacterial impacts without adversely affecting its SBS for clinical usage.
1. INTRODUCTION
It is substantial to use orthodontic composites with convenient merits; they easily disconnect after the end of the therapy but are hard to debond through treatment. In addition, to fulfill the therapeutic purposes of orthodontic remedy, it is fundamental to have an adequate bond between dental enamel and orthodontic brackets [1]. At present, resin composite is broadly used for this target by orthodontists because of its fair cost and reduced bracket bonding period [1, 2]. It has been reported that among patients pointing to orthodontic remedy, at least one patient suffers from debonded brackets with a minimal prevalence of 0.6% to 28.3% [3]. On the other side, brackets should be detached after the end of treatment. Therefore, balance is needed as the bond strength of the orthodontic brackets should counter the forces set through the treatment but not harm the tooth during the detaching process [4]. The shear bond strength SBS assay is an estimation step used for checking dental adhesives [5]. Numerous studies have demonstrated that SBS can be linked to abundant factors such as types of brackets and their base design and size, as well as type of etching material, adhesives, and restoration materials [6-8].
Unfortunately, orthodontic brackets, which are connected to enamel, result in their demineralization which elevates the risk of plaque accumulation and makes brackets’ connecting sites hard to be cleaned [9]. Classical use of antibacterial mouth rinses can reduce enamel demineralization [10]. Parallel to the previous concept, if a convenient antibacterial material is used to bind the brackets, demineralization around the bracket might be decreased [11]. Recently, the use of specialized nanoparticles (NPs) as antibacterial agents in the fields of medicine and dentistry has grown, as the addition of nanoparticles to orthodontic adhesives or cement may aid in inhibiting microbial attachment and enamel demineralization [12]. The physicochemical nature of the NPs enables them to interact with the negatively charged surface of bacterial cells to a greater extent. Research has reported that nanofillers minimize enamel deminera- lization without altering mechanical characters [13, 14]. For instance, composites comprising chitosan with hydroxyapatite nanoparticles prohibit the growth of Streptococcus salivarius, Streptococcus mutans, and Enterococcus faecalis and have a significant antibacterial activity [15].
Nanoparticles (1-100 nanometers) have been growing in dental fields such as orthodontics, implants, restorative materials, and periodontal illnesses [16-18]. The physical and mechanical merits of composites could be enhanced when nanoparticles were added [18]. Abundant studies have exposed the benefits of adding nanoparticles to composite resins concerning the antimicrobial merits and SBS of orthodontic composite resins [19-21]. Akhavan et al. [17] revealed the improvement of the SBS related to orthodontic adhesives when bonded with hydroxyapatite and silver nanoparticles. Also, the addition of silver nanoparticles to dental resin enhanced antimicrobial performance. The concentration and dispersion of nanofiller particles into the orthodontic adhesives are significant parameters that impact the antibacterial characters and their SBS. Frequently, the addition of various concentrations of NPs to the orthodontic adhesives elevated the antibacterial merits but decreased the SBS [17].
Titanium dioxide (TiO2) is one of the preferred materials for the antibacterial mission due to its color, biostability, compatibility, and chemical stability. Additionally, the continuous production of superoxide ions and hydroxyl radicals can decompose organic compounds [22, 23].
Table 1.
| ARI Score | Group 1 | Group 2 | Group 3 | Group 4 | Total |
|---|---|---|---|---|---|
| Titanium tetra isopropoxide (TTIP) | Sodium dihydrogen phosphate (Na2HPO4) | Titanium dioxide nanoparticles | Sodium tripolyphosphate | Mueller-Hinton agar | Whatman No. 1 filter paper |
| Ethanol | Calcium chloride (CaCl2) | Salvadora persica “Miswak” | Sodium tripolyphosphate | Tween 80 | Petri dish |
| Potassium bromide | Sodium hydroxide (NaOH) | Hydroxyapatite | Chitosan | - | - |
The current ex vivo study was planned to compare the SBS of orthodontic brackets attached to an orthodontic composite comprising titanium dioxide nanoparticles with that comprising chitosan and hydroxyapatite nano- particles. The null hypothesis was that the shear bond strength of a composite comprising titanium dioxide nanoparticles is not less than those of chitosan and hydroxyapatite nanoparticles. Table 1.
2. MATERIALS AND METHODS
2.1. Ethics Approval
The ethical code followed the guidelines of the National Committee of Bioethics, King Abdulaziz City for Science and Technology, Saudi Arabia, under the number REC40/3-098 [15].
The study involving human participants was conducted in accordance with the Helsinki Declaration of 1975, revised in 2013.
2.2. Preparation of Green Synthesized Titanium Dioxide Nanoparticles (TiO2 NPs)
The first 0.1 N of titanium tetra isopropoxide (TTIP) (Avra Laboratories Private Ltd, Hyderabad, India) is dissolved in twenty ml of ethanol solution under continuous stirring for thirty minutes. After that, a few drops of distilled water were added to constitute a dispersion medium. The mixture was set on the ultrasonic bath for twenty minutes, and then the sonicated solution was autoclaved at 150 °C for 3 h. Then, to remove the impurities, the sterile solution was cooled to room temperature, washed with deionized water, and centrifuged. The centrifuged solution was filtered with the Whatman No. 1 Filter paper (Merck, New Jersey, United States) [24]. On the other side, The Salvadora persica “Miswak” was previously gathered from Arak trees in Jazan City, Saudi Arabia, and then identified in the Faculty of Science, Jazan University with the specimen number JU/COP/19–7. The filtration of ten grams of leaves extract was performed through a 0.45mm filter, and the leaves were chopped, dried, and boiled in one hundred milliliters of water. The aqueous leaf extraction had a final concentration of 0.01775 g/mL [15].
For green synthesized nanoparticles; fifty millimeters of the filtered TTIP solution was added to twenty ml of the leaf extract drop by drop. The mixed solution was stirred for 3 hours at room temperature. The color of the solution was altered from pure white to yellowish-grey, indicating the formation of TiO2NPs. After that, the solution was dried at 110 °C for five hours. Then, the dried product was calcined in a Muffle furnace at 500 °C for two hours [25].
2.3. Characterization of TiO2NPs
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
The active functional groups of the synthesized titanium dioxide nanoparticles were estimated by FTIR (PerkinElmer, Germany) spectrophotometer, and about two milligrams of TiO2NPs were loaded with potassium bromide pellets (Merck, New Jersey, United States). The sample was scanned in the range of 400–4000 cm−1 at a resolution of 4 cm−1 [26].
2.3.2. Energy-dispersive X-ray Spectroscopy (EDX)
It is employed to determine TiO2NPs homogeneity and their elemental dispersal in the examined compound. About ten microliters of TiO2 NPs were dropped on a carbon stub and let to air dry. The EDX-JEOL JSM 6360 (JEOL, Ltd., Tokyo, Japan) was operated at 20 kV for 19s, and the data on elements present in the sample was gathered [27].
2.4. Preparation of Chitosan and Hydroxyapatite Nanoparticles
The chitosan nanoparticles used for comparison were formerly synthesized by the ionic gelation method and characterized as described [15, 28]. In detail, incor- poration of Salvadora persica was added to sodium tripolyphosphate (Merck, New Jersey, United States), then the extract mixture was added drop-wise to chitosan nanoparticles, the mixture was dialyzed via spectra/por-cellulose membrane; Mw 3000 Da for removing any free plant. One percent Tween 80 (Merck, New Jersey, United States) was added in magnetic stirring for 2 hours, and then the chitosan particle suspension was centrifuged for 30 min at 12,000xg. The pellet was resuspended in deionized water and freeze-dried till use.
Similarly, the hydroxyapatite nanoparticles from Salvadora persica plant extract also were also formerly synthesized and characterized as described [15, 29]. In brief, the mixture of 0.6 M sodium dihydrogen phosphate (Na2HPO4) and 1 M calcium chloride (CaCl2) was then adjusted at pH above 10 using 0.7 M sodium hydroxide (NaOH); all chemicals were obtained from Sigma-Aldrich, St. Louis, MO, USA. The prepared and filtered plant extract was used as a solvent, and then the previous mixture was added to obtain a white precipitate, which was then washed with deionized water.
2.5. The Ex-vivo Study
2.5.1. Specimen Preparation
This ex-vivo study was conducted on forty healthy human premolars extracted from patients who had dental histories of no chemical use. Removal of soft tissue debris from teeth samples, as well as a re-examination of all enamel surfaces, was performed using a stereomicroscope (Nikon, Tokyo, SMZ 1500, Japan). The samples were conserved at room temperature in distilled water till experimentation [30]. Teeth samples were cleaned, washed, and dried, then vertically mounted in acrylic cylinders in such a way that their crowns were presented [31].
The buccal surface of the tooth was etched using 37% phosphoric acid (Sigma-Aldrich, St. Louis, MO, USA) for thirty seconds, rinsed thoroughly with running water for thirty seconds, and gently dried with air spray. A thin coat of primer was then applied with an applicator tip and light-cured for ten seconds, persuaded with the three types of orthodontic adhesives, light-cured for forty seconds (ten seconds on each side). While bonding the brackets to the tooth surface, a three hundred grams force was applied for approximately five seconds to assert a uniform thickness of the adhesive. Excessive adhesive was eliminated with a probe. After completion of the bonding steps, the teeth were immersed in a container with distilled water for twenty-four hours. The forty teeth samples were randomly portioned into four uniformed groups (n ≈10 in each group) relying on the type of composite comprising Group 1: brackets bonded in composite without nanoparticles. Group 2: the composites contain TiO2NPs. Group 3: the composites comprise chitosan nanoparticles. Group 4: the composites comprise chitosan with hydroxyapatite nanoparticles.
2.5.2. Assessment of Shear Bond Strength
All forty teeth samples were thermo-cycled 1500 times between 5 to 55 Cᵒ, with fifteen seconds in each temperature and ten-second intervals for sample aging intentions. After that, the samples were cut and rode in the self-cure resin for better constancy, and then shear forces were implemented on brackets of all four groups using a universal testing machine till the bond failure occurred and shear bond strength (SBS) was calculated in megapascals (MPa). The findings of this study were compared with the recommended bond strength of 5.9–7.8 MPa suggested by Reynolds [32]
2.6. Adhesive Remnant Index (ARI) Scoring
After debonding, the exterior enamel surface was examined using a light stereomicroscope (Nikon SM2-10, Japan) at ×10 magnification to define the amount of residual adhesive and scores for each tooth through the prevalent used ARI scale as referred [33, 34]. The three-scaled scoring process is one of the most commonly used indices in orthodontic adhesive examination, and it quantifies adhesive left on the tooth.
Score 0: less than 10% of the adhesive remained on the tooth.
Score 1: between 10 and 50% of residual adhesive left.
Score 2: between 50 and 90% of residual adhesive left.
Score 3: more than 90% of the residual adhesive left on the tooth with the impression of the bracket base.
The ARI score was employed to determine the site of bond failure between the adhesive, enamel, and the bracket base.
2.7. Antibacterial Activity of TiO2NPs
The antibacterial performance of TiO2NPs was assessed using the agar diffusion method, which tests the ability of antibacterial components to diffuse within agar and induce a bacterial prohibition zone. First, the Mueller-Hinton agar (Sigma-Aldrich, St. Louis, MO, USA) was poured onto the Petri dishes. For studying the inhibition zone, the six mm diameter wells were filled with fifty microliters from the bio-formed TiO2NPs of different ten-folded dilutions. Twenty-four hours broth cultures of Escherichia coli O25: K11 and Staphylococcus intermedius medium were adjusted to 108 CFU /ml using a spectrophotometer and 20 µL inoculated onto the plates then incubated aerobically at 37 °C for 24 to 48 hrs. The inhibition zone that indicated the antibacterial activity of TiO2NPs was estimated in millimeters using an inhibition zone measuring scale [26].
2.8. Statistical Analysis
Shear bond strength results were analyzed using debonding force measured in Newton (N) and altered to mega-Pascal (MPa) by portioning to the surface area of the bracket base. The bond strengths were estimated at a crosshead speed of 1 mm/minute, as the standard deviation values were not less than half of the mean value, which indicates the data did not persuade normal distribution. The results of shear bond strength (SBS) values in MPa were analyzed using SPSS 21 software via one-way ANOVA and Tamhane's T2 multiple comparison tests.
The data of the antibacterial tests were analyzed using SPSS 21 software (IBM®, Chicago, USA) via one-away ANOVA, which was employed to compare the variations in shear bond strength between and within the four groups. Notably, in all statistical assays, the significance p-value was considered when lower than 0.05 at a 95% confidence interval.
3. RESULTS
3.1. Characteristics of Titanium Dioxide Nanoparticles
The optical behavior of the titanium dioxide nanoparticles was examined via the DRS. The UV–visible reflectance spectrum of TiO2 nanoparticles is displayed in Fig. (1).
The prepared TiO2 NPs were examined by the FT–IR spectrum to identify the presence of chemical compounds and functional groups. The FT–IR spectrum of TiO2 NPs is demonstrated in Fig. (2). The defined band referred to C–O groups of aromatic stretching vibration. The strong bands at 460 cm-1 and 900 cm-1 demonstrated the synthesis of Ti–O and Ti–O-Ti bending vibrations, respectively. Peaks noticed at 460–900 cm-1 may disappear/partially reduce in intensity by annealing temperature.

The UV–Visible reflectance spectrum of TiO2 NPs derived from aqueous leaf extract of Salvadora persica.
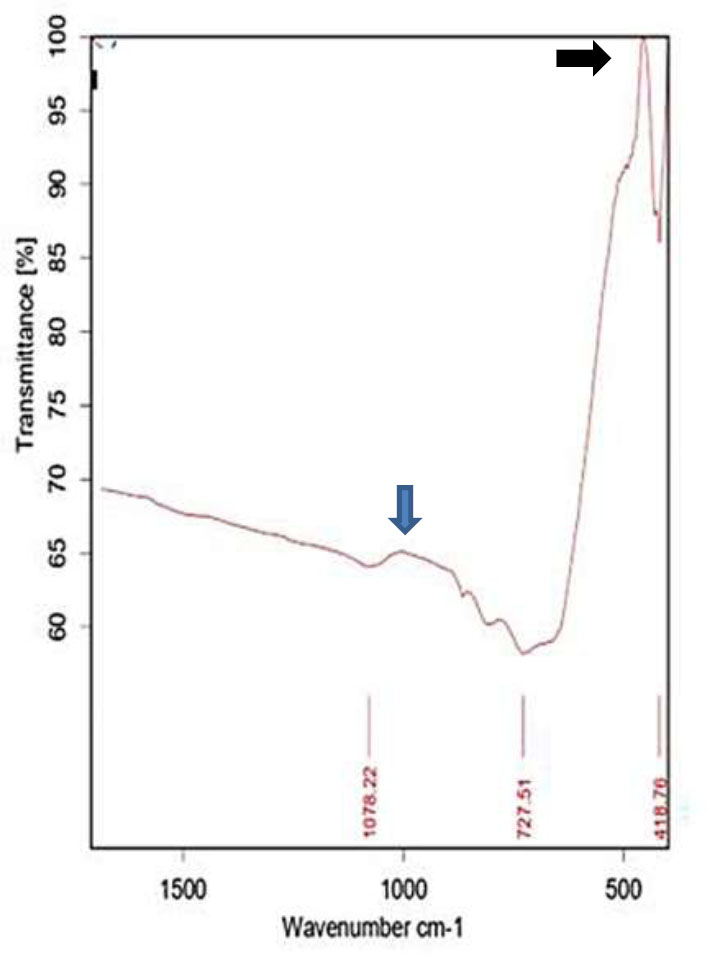
FT–IR spectra of TiO2 nanoparticles: the black arrow pointed to the Ti–O band peak, while the blue arrow pointed to the Ti–O-Ti band peak.

TEM micrograph of formed TiO2 NPs.
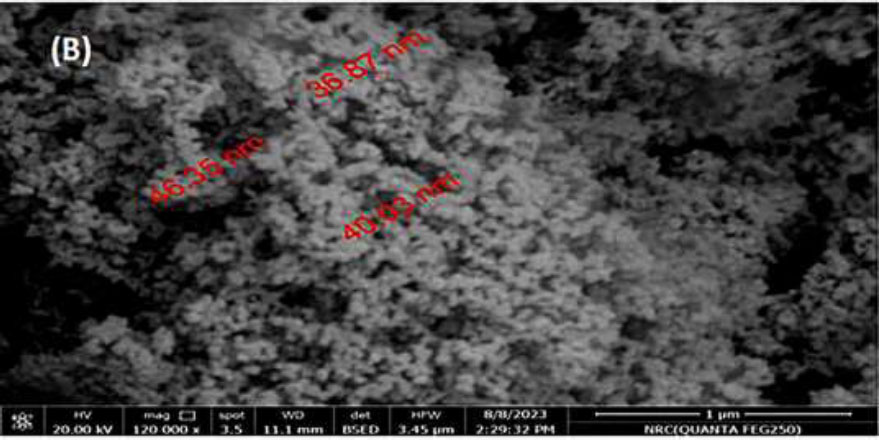
SEM micrograph of formed TiO2 NPs.
| Element | Weight % | Atomic % | Net Int. |
|---|---|---|---|
| CK | 13.81 | 23.81 | 48.42 |
| OK | 45.13 | 58.43 | 103.14 |
| TIK | 41.06 | 17.75 | 517.09 |
The TEM and SEM display of bio-formed TiO2 NPs is shown in Figs. (3a and b), respectively.
On the other hand, the EDX spectrum was applied to the obtained bio-mediated TiO2 NPs to declare the elemental analysis of the chemical components. The EDX spectra were shown in Fig. (4) and displayed that the elements that exist in the formed TiO2 NPs are titanium (Ti) at 41.06%, oxygen (O) at 45.13%, and carbon (C) at 13.81%. The atomic composition and weight % of the TiO2 nanoparticles are mentioned in Table 2.
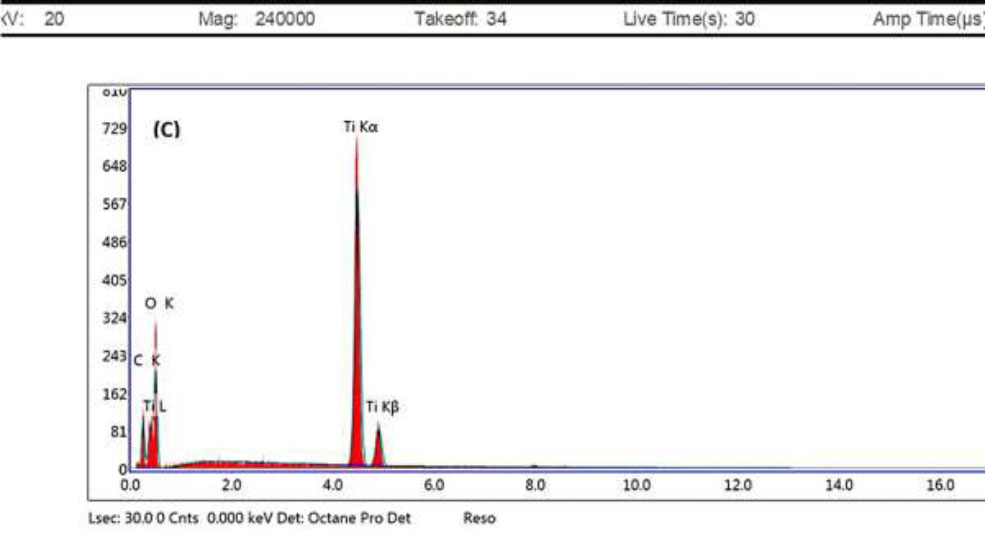
EDX profile of bio-mediated TiO2 NPs.
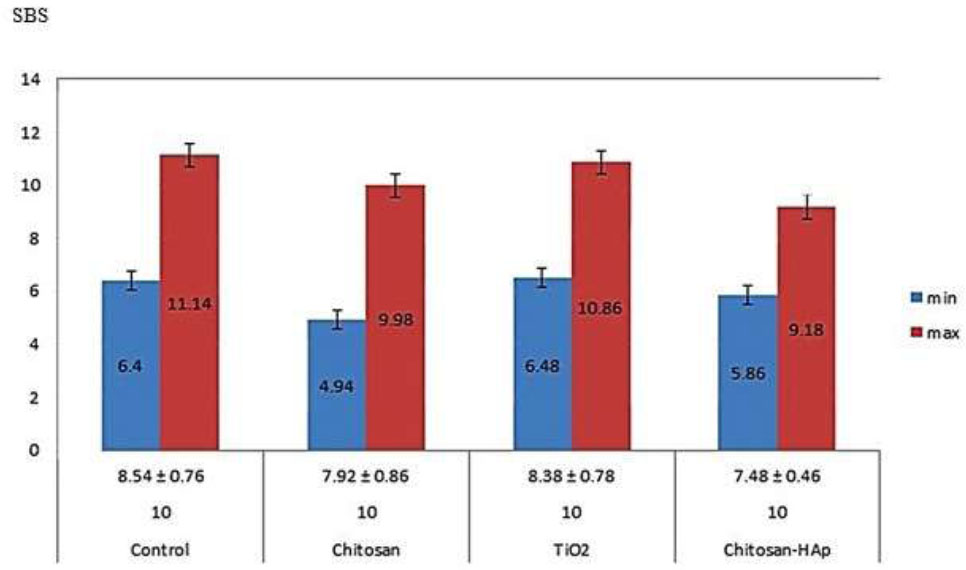
Shear bond strength values in the examined groups. The significance p-value level was considered when it was lower than 0.05.
| ARI Score | Group 1 | Group 2 | Group 3 | Group 4 | Total |
|---|---|---|---|---|---|
| ARI 0 | 2 | 2 | 2 | 1 | 7 |
| ARI 1 | 7 | 5 | 3 | 3 | 18 |
| ARI 2 | 1 | 2 | 3 | 3 | 9 |
| ARI 3 | 0 | 1 | 2 | 3 | 6 |
| Total | 10 | 10 | 10 | 10 | 40 |
3.2. The Shear Bond Strength (SBS)
The SBS values and descriptive statistics for all examined four groups are displayed in Fig. (5). Based on the obtained data, the control group revealed more mean shear bond strength (8.54 ± 0.76) in parallel to the three groups containing nanoparticles incorporated composites. Interestingly, the group comprising TiO2 NPs exhibited the nearest mean value to the control group (8.38 ± 0.78), which was higher than the other two groups; chitosan (7.92±0.86) as well as chitosan and hydroxyapatite (7.48 ± 0.46). At the same time, there were insignificant variations according to the One-Way ANOVA test for all four studied groups (p= 0.379).
3.3. Adhesive Remnant Index (ARI) Scoring
The ARI scores results were noticed in 18 teeth through the 4 groups, with score 1 as 45% followed by score 2, score 0, and score 3 as 22.5%, 17.5%, and 15%, respectively, as shown in Table 3. The p-value from the one-way ANOVA is approximately 0.171. This suggests that there is no significant difference in the mean ARI scores between the four groups at a 0.05 significance level. Additionally, using The Mann–Whitney test, all groups comparing were non-significant except group 1 vs. group 4 = 0.0379.

Shows the antibacterial activity of TiO2 NPs activity. The significance p value level was considered when lower than 0.05.
3.4. The Antibacterial Performance of TiO2 NPs
The antibacterial performance of TiO2 NPs was investigated against Gram-negative and Gram-positive bacteria and demonstrated in Fig. (6). The minimal bactericidal concentrations (MBC) were 7g/mL and 5g/mL obtained at 1x103 and 1x105 against E. coli and Staphylococcus intermedius strains, respectively. The obtained results were compared with rifampicin (RA) (30 mg L−1) antibiotic as the positive control.
4. DISCUSSION
It is shown that the spectrum of TiO2 NPs peaked at 393 nm, pointed the charge-associated electronic transition between the Ti at the 3d state and the O2p state. Through biosynthesis, the sharp absorption peak coincides with the alteration in the crystalline stage and the average crystalline size. Thus, the examined nanomaterial is applicable for catalytic implementation. The acute absorbance peak around 300–400 nm region asserted the synthesis of TiO2 nanoparticles. The phytochemical components such as alkaloids, phenolic acids, proteins, tannins, terpenoids, and others could minimize and stabilize the complex metals into simple elements [35]. At the time of addition of the plant extract to the titanium dioxide salt, the reduction steps were initiated instantly by the plant extract with color changes from white to yellowish-grey, indicating the synthesis of TiO2 NPs. Various ranges of optical behavior peaks corresponding to the TiO2 NPs in UV–vis spectroscopy were reported formerly using different plant extracts in other previous investigations [36-39].
The metal oxide bonds as Ti–O and Ti–O–Ti emphasized the presence of TiO2 in the formed TiO2 NPs. The existence of the Ti–O–Ti bond indicated the stability of TiO2 in green synthesis because of the strong interaction of biomolecules as alkaloids, coumarins, flavonoids, tannins, and terpenoids which are responsible for reducing the bulk of TiO2. The displayed FTR examination was harmonized with other studies [40-43].
The synthesized TiO2 NPs are spherical-shaped structures with an average particle size of 8-47 nm. The lower particle size is usually inversely proportional to the surface volume. Therefore, the decrease in particle size initiates fast penetration to the bacterial surface and toxic elements. The size and shape of the TiO2 NPs could be defined by the nature of the reducing agents employed and the exposure time. The SEM pattern in our study corresponded to other investigations [44, 45].
These results suggested that the obtained particles were partially purified. Additionally, the titanium element was highly paralleled to oxygen content, and this pattern is usually observed in bio-mediated TiO2 NPs [40].
The result of our SBS analysis coincided with other studies that addressed the same methods [46, 47]. Great agreement was observed with the data obtained by Mahendra et al. [48], who mentioned that the control group exposed the highest SBS, persuaded by titanium and other groups, with statistically significant variation in mean SBS values among all groups. Felemban and Ebrahim [33] mentioned that the addition of TiO2 nanoparticles to orthodontic adhesive elevated shear bond strength, tensile strength, and compressive strength in vitro.
Reynolds [31] admonished minimum bond strength of 5.9–7.8 MPa as sufficient bond strength for most orthodontic needs along with routine clinical use. The bond strength value of the TiO2, chitosan, as well as chitosan and hydroxyapatite nano-adhesive used in the current study was over this minimum requirement and gave clinically acceptable mean SBS.
Our ARI findings coincided with other previous studies [49-52] as no significant variations were observed in ARI scores based on the kind of composite or addition of TiO2 (p>0.05).
However, it should be considered that shear bond strength and ARI scores can also be influenced by other factors such as conditioning type [53], surface design [54], and enamel contamination [55]. Therefore, future research is needed to test these concerns.
The cell wall of the Gram-negative bacteria contains a thin layer of peptidoglycan, while a thicker layer is present in Gram-positive bacteria. The antibacterial activity of TiO2 NPs was tested versus human pathogens: Escherichia coli O25: K11 and Staphylococcus intermedius. The higher zone inhibition layer was noticed in E. coli relative to S. intermedius, and this observation may be attributed to the thin walls of Gram-ve bacteria, which are quickly broken by the positive ions of TiO2NPs due to the electrostatic interaction that existed between the positive TiO2 nanoparticles and the negatively charged cell wall surface of E. coli which resulted in a high prohibition zone on Gram-ve bacteria [56].
It was found that the zone of inhibition of formed TiO2NPs exhibited excellent antibacterial performance and was poisonous to pathogens. Thus, the synthesized TiO2NPs are greatly applicable to numerous biomedical applications [51, 53, 57]. A very recent study performed by Elabd et al. [58] stated that the addition of 1% TiO2NPs to orthodontic functional acrylic baseplates significantly decreased the bacterial colony count beneath the base plate after at least 4 months of application.
CONCLUSION
In this current study, TiO2 NPs are successfully formed by green synthesis via the aqueous leaf extract of Salvadora persica “Miswak.” Color alterations asserted the reduction of bulk titanium to nanoparticles by a one-step and eco-friendly technique. Scanning electron microscopy and micrograph observed a homogenous spherical shape surface appearance. The SBS values were not significantly different across all four studied groups. The antibacterial performance of TiO2 NPs was tested versus bacterial pathogens: Escherichia coli O25: K11 (Gram -ve bacteria) and Staphylococcus intermedius (Gram +ve bacteria). The bio-mediated various concentrations of TiO2 NPs demonstrated a potent antibacterial performance. The results revealed the merit of TiO2 NPs as a prospect for incorporating orthodontic composites. The mass production and purification possibilities of these TiO2 NPs are in growing progress.
AUTHORS' CONTRIBUTIONS
A.S.A.: Conceptualized and designed the research work, acquired data, and statistics, and drafted as well as formatted the manuscript; T.E.: Prepared the green synthesized titanium dioxide nanoparticles (TiO2 NPs); T.E. and A.A.: Performed the characterization of TiO2 NPs.
LIST OF ABBREVIATIONS
| SBS | = Shear bond strength |
| NPs | = Nanoparticles |
| TiO2 | = Titanium dioxide |
| TiO2 NPs | = Titanium dioxide NanoParticles |
| TTIP | = Titanium tetra IsoPropoxide |
| FTIR | = Fourier Transform Infrared Spectroscopy |
| EDX | = Energy-Dispersive X-Ray spectroscopy |
| SEM | = Scanning Electron Microscopy |
| TEM | = Transmission Electron Microscopy |
| DRS | = Diffuse Reflectance Spectrophotometer |
| Na2HPO4 | = Sodium dihydrogen phosphate |
| CaCl2 | = Calcium chloride |
| NaOH | = Sodium hydroxide |
| ARI | = Adhesive Remnant Index |
| MPa | = Mega-Pascal |
| ANOVA | = Analysis of Variance |
| MBC | = Minimal Bactericidal Concentrations |
| E. coli | = Escherichia coli |
| S. intermedius | = Staphylococcus intermedius |
| RA | = Rifampicin |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study design was carried out and followed the instructions of the National Committee of Bioethics, King Abdulaziz City for Science and Technology, Saudi Arabia under the number REC40/3-098.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.


