All published articles of this journal are available on ScienceDirect.
Exploring the Potential Clinical Applications of Salivary Cortisol in the Diagnosis and Management of Cushing’s Syndrome, Diabetes, Depression, and Periodontal Disease: A Systematic Review
Abstract
Background
Current research primarily aims to investigate the potential of salivary cortisol for early diagnosis as well as clinical management and monitoring of disease progression. Its utility extends to a range of multidisciplinary settings, encompassing conditions, such as Cushing's syndrome, stress, and depression, pre-diabetes, type 2 diabetes, and periodontal disease, within dentistry. This systematic review aimed to analyze recent literature on the use of salivary cortisol as a biomarker for various clinical and pre-clinical conditions, including stress, depression, diabetes, Cushing's Syndrome (CS), and periodontal disease. Specifically, the review sought to evaluate its application in screening, diagnosis, clinical management, and monitoring disease progression.
Materials and Methods
Employing PubMed (MEDLINE) and Scopus databases, the search strategy utilized Medical Subject Heading (MeSH) terms, including “Cushing’s syndrome”, “diabetes mellitus type 2”, “hydrocortisone”, “saliva”, “biomarker”, “depression”, and “periodontal disease”, following the PICO model. The Cochrane Collaboration tool was used to assess bias risk for randomized clinical studies, while the ROBINS-I tool was used for observational studies.
Results
Adhering to PRISMA guidelines, 25 studies, comprising controlled interventions, pre-post studies, and observational/cohort or cross-sectional studies, were analyzed. We found a correlation between salivary cortisol levels and various health conditions. Elevated salivary cortisol was associated with increased disease severity in periodontitis, characterized by higher probing pocket depths and a greater plaque index. Patients with chronic periodontitis exhibited notably higher cortisol levels compared to healthy individuals, suggesting a link between stress and inflammatory responses in periodontal disease. Furthermore, salivary cortisol was identified as a valuable biomarker for detecting conditions, such as Cushing's syndrome and type 2 diabetes, with altered cortisol patterns indicative of disease progression. The findings highlighted the potential of salivary cortisol as a non-invasive diagnostic tool in assessing health status and managing related conditions.
Conclusion
Salivary cortisol serves as a crucial biomarker for the diagnosis and management of several health conditions, including Cushing's disease, diabetes, stress, depression, and periodontal disease. Its ease of measurement and reliability provide valuable insights into the Hypothalamic-pituitary-adrenal (HPA) axis, and the standardization of testing methods could enhance diagnostic accuracy. Continued research is essential to elucidate the interconnections among these conditions, which may inform future diagnostic and therapeutic strategies.
1. INTRODUCTION
Cortisol, also known as 17-hydroxy-corticosterone, is a steroid hormone produced by the adrenal cortex. It plays a crucial role in regulating and maintaining overall health, impacting various systems, including cardiovascular, cognitive, immune, metabolic, musculoskeletal, respi- ratory, and reproductive systems, as its receptors are found in multiple cell types [1, 2]. Cortisol is the product of the Hypothalamic-pituitary-adrenal (HPA) axis. This signaling pathway originates from the hypothalamus, which receives neurological inputs from the Para- Ventricular Nucleus (PVN) and the hypothalamic suprachiasmatic nucleus. In response to triggers, PVN cells secrete Corticotropin-releasing Factor (CRF), which stimulates the release of Adrenocorticotropic Hormone (ACTH) from the pituitary gland. ACTH then enters the bloodstream, exerting a steroidogenic effect and triggering the adrenal cortex to release cortisol. Cortisol levels typically peak in the morning, around 30-50 minutes after waking up, and gradually decline throughout the day, following a circadian pattern, with the lowest levels occurring at night time [3, 4].
Several systemic conditions are related to altered levels of cortisol. Hypercortisolism presents as a clinical state where tissues endure extended exposure to steroid hormones, notably cortisol, which may result in hyper- cortisolemia. Prolonged hypercortisolism can precipitate the onset of Cushing's Syndrome (CS). CS may be induced by sustained systemic exposure to a spectrum of glucocorticoids, whether originating externally or internally [5]. Stress-related disorders can emerge from an overactive Hypothalamic-Pituitary-Adrenal (HPA) axis, influenced by prolonged negative expectations about future stressors. Individuals with heightened anticipations tend to mentally prepare for stressful events, prolonging activation of the dorsolateral prefrontal cortex while suppressing the amygdala. This dampening of the amygdala's response diminishes activation of the HPA axis, potentially exacerbating stress-related conditions [6].
Type 2 Diabetes Mellitus (T2DM), a prevalent metabolic disorder, results from the combined effects of impaired insulin secretion by pancreatic β-cells and the diminished responsiveness of insulin-sensitive tissues to insulin [7]. Excessive cortisol levels, whether clinically evident or experimentally induced, are linked with significant disruptions in intermediate metabolism, manifesting as abdominal obesity, insulin resistance, and reduced levels of HDL-cholesterol, ultimately increasing the risk of developing diabetes [8].
Chronic Periodontitis (CP) is a multifactorial inflammatory disease that primarily targets the supportive structures of the teeth, including the alveolar bone, periodontal ligament, and gingiva. This condition not only results from a microbial insult, but is also significantly influenced by various systemic factors, including hormonal changes that play a crucial role in regulating bone metabolism and immune responses. Hormonal fluc- tuations, particularly those involving cortisol, estrogen, and parathyroid hormone, contribute to the delicate balance between bone resorption and formation, which is essential for maintaining periodontal health [9, 10].
Elevated levels of pro-inflammatory cytokines, such as Interleukin-1 beta (IL-1β) and Tumor Necrosis Factor-alpha (TNF-α), are strongly associated with periodontal tissue destruction. These cytokines promote the recruitment and activation of osteoclasts, leading to increased bone resorption in the periodontal environment. When hormonal dysregulation occurs, it can exacerbate the inflammatory response, further promoting the production of these cytokines and accelerating periodontal breakdown [9, 10].
Cortisol, a glucocorticoid hormone known for its immunosuppressive effects, is produced in response to stress and plays a vital role in modulating immune responses. In the context of periodontitis, elevated cortisol levels can suppress the host’s immune function, impairing its ability to effectively respond to bacterial challenges within the periodontal pocket. This immunosuppressive effect of cortisol can lead to a reduced capacity to control bacterial invasion, thereby exacerbating inflammation and accelerating bone loss in affected areas [11].
Estrogen is another hormone that significantly impacts bone metabolism. It is known for its protective effects on bone density and structure, especially in women. Decreased estrogen levels, such as those seen in postmenopausal women, are associated with increased bone resorption, which heightens the risk of periodontal tissue degradation. The deficiency of estrogen leads to an imbalance in bone turnover, favoring osteoclastic activity over osteoblastic activity, thus accelerating bone loss. This increased susceptibility in postmenopausal women highlights the need to consider hormonal status as a factor in the risk assessment and management of periodontal disease [12].
The regulation of bone homeostasis within the oral cavity is a complex process influenced by the interplay of several hormones, including cortisol, estrogen, and parathyroid hormone. Each of these hormones has a distinct role in modulating both osteoclastic (bone-resorbing) and osteoblastic (bone-forming) activities. Parathyroid hormone, for example, influences calcium metabolism and stimulates bone remodeling, which can impact the structural integrity of the alveolar bone. Together, these hormones maintain a dynamic equilibrium essential for bone health, and any disruption in their levels can predispose individuals to bone-related diseases, including periodontitis [13].
The onset and progression of periodontitis are, therefore, not solely attributable to microbial factors, but are also influenced by various systemic conditions that affect the host's immune and inflammatory responses. Chronic stress, diabetes, and smoking are recognized as significant contributors to the pathogenesis of periodontal disease. Chronic stress is particularly detrimental because it leads to prolonged elevation of cortisol levels, which can exacerbate inflammatory processes and impair immune function within the oral cavity. This prolonged exposure to cortisol creates an environment conducive to periodontal degradation by weakening the host's defenses against bacterial colonization [14, 15].
Moreover, diabetes is another systemic condition closely linked with periodontal disease. It is associated with an altered inflammatory response, characterized by an exaggerated release of pro-inflammatory mediators, which not only increases the susceptibility to periodontal destruction, but also compromises the healing processes within periodontal tissues. The bidirectional relationship between diabetes and periodontitis emphasizes the importance of managing systemic conditions to prevent exacerbation of periodontal disease and promote overall oral health [15].
In the past, cortisol levels were determined by testing blood and urine samples before Kirshbaum recognized saliva samples as a valid alternative in clinical diagnosis [16]. This transition revolutionized the assessment of cortisol secretion, shifting from a clinical setting to a multidisciplinary approach [17]. Salivary cortisol measure- ments are considered accurate because cortisol passively flows through the salivary glands, and its concentration is not dependent on salivary volume. It serves as a reliable tool for measuring biologically active cortisol, reflecting the cortisol flowing through the bloodstream at any given moment [18, 19]. This biomarker is widely applied in medical and biological fields, providing valuable information about the HPA axis, both in health and disease, and its implications for human responsiveness and adaptation to environmental changes [20]. In field-based research, various cortisol measures are widely utilized and closely linked to physiological phenomena (Table 1) [21]. There is a growing consensus among researchers regarding the significance of diurnal fluctuations in cortisol levels. Both the Cortisol Awakening Response (CAR) and diurnal slope are recognized as crucial components in assessing diurnal cortisol activity [21].
| Cortisol Measures | Description |
|---|---|
| Cortisol Awakening Response (CAR) | Captures the cortisol surge 30-45 minutes after waking [17] |
| Diurnal cortisol slope | Tracks cortisol level changes (typically declining) from morning to evening [25] |
| Area Under the daytime cortisol Curve (AUC) | Signifies cumulative cortisol levels during the day [26] |
| Waking cortisol | Reflects the cortisol level upon waking up [25] |
| Cortisol measured at specific waking times | Analyzes cortisol levels at distinct clock points upon waking [27] |
| Bedtime cortisol | Indicates cortisol levels at night [25] |
| Cortisol reactivity to momentary stressors | Gauges the rise above normal levels for a specific individual in response to immediate stressors [28] |
| Cortisol reactivity to daily stressors | Assesses variations in cortisol levels from day to day in response to daily stressors [28] |
Over time, research has concentrated on salivary cortisol as a biomarker linked to various conditions. In the literature, it has been shown that elevated IL-6 levels are correlated with reduced CAR, increased Area Under the Curve (AUC), and flatter diurnal cortisol decline. Higher TNF-α levels were linked to lower waking cortisol levels, independent of IL-6 and IL-10. No significant associations were found between IL-10 and cortisol parameters [22]. Concerning salivary α-amylase, it increases in response to stress, reflecting nervous system activity. It is emerging as a diagnostic marker for oral conditions, underlining its association with cortisol in stress responses [23]. Moreover, if cortisol is linked to autoimmune diseases, rheumatoid arthritis patients may exhibit higher levels of plasmatic cortisol compared to healthy subjects [24].
Therefore, this review aimed to analyze recent literature on salivary cortisol as a biomarker for several clinical and pre-clinical conditions, including stress, depression, diabetes, CS, and periodontal disease, as well as assess its application in screening, diagnosis, clinical management, and monitoring disease progression.
2. MATERIALS AND METHODS
2.1. Focused Questions
The questions included the following: Is salivary cortisol a clinically applicable biomarker for the diagnosis, management, and progression of Cushing’s syndrome, diabetes, depression, and periodontitis? Can salivary cortisol be used for the detection of pre-clinical conditions (hypercortisolism, prediabetes, stress)?
2.2. Eligibility Criteria
The inclusion criteria applied in this review were as follows: (I) study model: interventional studies, retrospective studies, cross-sectional studies, and cohort studies; (II), participants: patients with Cushing’s syndrome, pre-diabetes/type 2 diabetes, stress/depression, periodontitis; (III) interventions: salivary cortisol measurements for screening, clinical management, and disease progression; and (IV) outcome: the reliability of salivary cortisol in screening, diagnosis of pre-clinical and clinical conditions, clinical management, and monitoring disease progression.
Following the methods of Scribante et al. [29], exclusively studies adhering to all the inclusion criteria were examined. With respect to the exclusion criteria, the following were considered: (I) abstract of articles published in non-English languages, (II) duplicate studies, (III) not relevant studies (full-text articles not appropriate to answer the focused question, analysis of different supplementary treatments, full-text content not corresponding to abstract), (IV) ex vivo or experimental animal studies, (V) absence of ethics committee approval, (VI) narrative reviews, systematic reviews, or systematic and meta-analysis reviews, and (VII) case report studies.
2.3. Search Strategy
The PICO model (Table 2) [30] (Population, Intervention, Comparison, Outcome) was used to conduct this review through a literature search of the PubMed (MEDLINE) and Scopus electronic databases, founded on the following three aspects: population (people with Cushing’s disease, pre-diabetes/diabetes, stress/ depression, periodontal disease), concept (evidence from clinical trials related to salivary cortisol levels and clinical application in diagnosis and management of the aforementioned conditions), and context (in this regard, the review has not been circumscribed to any specific cultural element or setting). Studies’ abstracts that analyzed the potential of salivary cortisol levels for screening, clinical management, and monitoring disease progression of several conditions and their predictive value for pre-clinical conditions were reviewed. During this scoping review of the literature, the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) consensus was followed (Table S1) [31].
| 1. Participants/population: patients with hypercortisolism/Cushing’s disease, pre-diabetes/diabetes, stress/depression, and periodontal disease. |
| 2. Intervention/exposure: salivary cortisol concentration for hypercortisolism/Cushing’s disease, pre-diabetes/diabetes, stress/depression, and periodontal disease’s early diagnosis and/or management. |
| 3. Comparison/control: no comparison. |
| 4. Outcome: the role of salivary cortisol levels in early diagnosis and/or management of hypercortisolism/Cushing’s disease, pre-diabetes/diabetes, stress/depression, and periodontal disease. |
2.4. Research
The Medical Subject Heading (MeSH) terms used are as follows: “Cushing syndrome”, “diabetes mellitus type 2”, “hydrocortisone”, “saliva”, “biomarker”, “depression”, and “periodontal disease”; an electronic search was performed on PubMed (MEDLINE) and Scopus databases [32, 33].
The search string applied for the search was built as follows: #1 “Cushing syndrome” (MESH) OR (syndrome, Cushing) OR (Cushing's syndrome) OR (syndrome, Cushing's) OR (hypercortisolism); #2 “diabetes mellitus, type 2” (MESH) OR (diabetes mellitus, noninsulin-dependent) OR (diabetes mellitus, ketosis-resistant) OR (diabetes mellitus, ketosis resistant) OR (ketosis-resistant) OR (diabetes mellitus) OR (diabetes mellitus, non-insulin dependent) OR (diabetes mellitus, non-insulin-dependent) OR (non-insulin-dependent diabetes mellitus) OR (diabetes mellitus, stable) OR (stable diabetes mellitus) OR (diabetes mellitus, type II) OR (NIDDM) OR (diabetes mellitus, noninsulin dependent) OR (diabetes mellitus, maturity-onset) OR (diabetes mellitus, maturity onset) OR (maturity-onset diabetes mellitus) OR (maturity onset diabetes mellitus) OR (noninsulin-dependent diabetes mellitus) OR (noninsulin dependent diabetes mellitus) OR (maturity-onset diabetes) OR (diabetes, type 2) OR (diabetes mellitus, adult-onset); #3 “hydrocortisone” (MESH) OR (cortisol) OR (epicortisol); #4 “saliva” (MESH) OR (salivas); #5 “biomarkers” (MESH) OR (biological markers) OR (biomarker) OR (serum markers) OR (clinical markers) OR (biochemical markers) OR (laboratory markers); #6 “depression” (MESH) OR (depressive symptoms) OR (depressive symptom) or (emotional depression) OR (depression, emotional); #7 “periodontal diseases” (MESH) OR (disease, periodontal) OR (diseases, periodontal) OR (periodontal disease) OR (parodontosis) OR (pyorrhea alveolaris); #8 combination of #1 AND #4 AND #5 ; #9 combination of #2 AND #4 AND #5; #10 combination of #3 AND #4 AND #7.
The articles published in the years 2010 to 2023 were selected. The data extraction period was between February 2024 and June 2024. The last search was performed on 30th June, 2024. Two calibrated reviewers (M.G. and M.P.) conducted the research.
Disagreements and discrepancies were resolved by consensus, and four other reviewers were consulted (F.P., A.S., A.B.G., and F.S.). Careful analysis of the titles and abstracts was conducted for the initially searched articles, ensuring that only relevant studies were included while non-relevant ones were excluded. All pertinent articles underwent thorough review and scrutiny by examining complete texts, documenting the discoveries, and identifying any similar studies that met the chosen inclusion criteria.
The present protocol has been registered within the Open Science Framework platform (registration DOI-10.17605/OSF.IO/P4KVB).
The elaborated strategies applied for each electronic database are exhibited in Table S2 (supplementary material).
3. RESULTS
The primary search identified 439 articles based on MeSH terms. Following this, 402 articles were removed (13 abstracts of articles published in non-English languages, 198 duplicates, 68 in vitro or animal clinical studies, 114 because they were not pertinent, and 9 because of the absence of ethics committee approval), and 37 articles were screened based on title and abstracts. The remaining 25 full-text articles were assessed for eligibility. Additionally, 12 full-text articles were further excluded because they were considered irrelevant. The 25 relevant articles were finally included and analyzed in this review. The flowchart of the review process is described in Fig. (1).
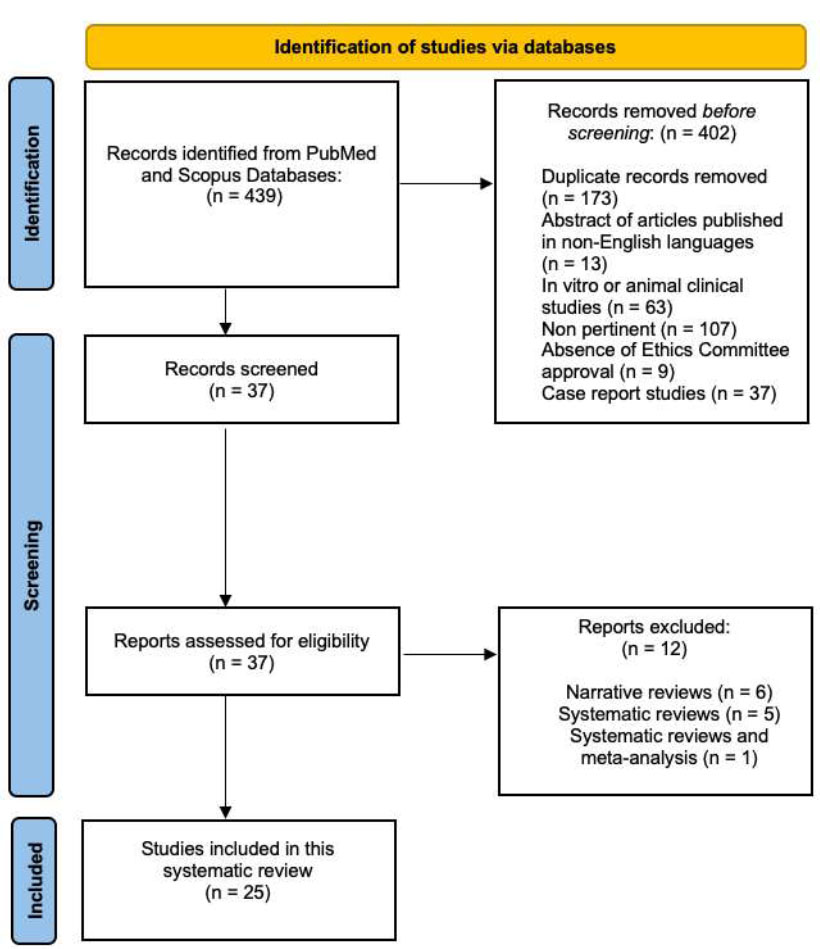
PRISMA 2020 flow diagram for systematic reviews.
Table S3 shows the studies excluded from this review and the reasons for exclusion [34-45].
The studies were from two categories: 2 controlled intervention studies [46, 47] and 23 observational/cohort or cross-sectional studies [6, 48-69].
3.1. Literature Review Results
In the 25 selected studies, salivary cortisol was linked to different conditions. From the 25 studies selected in this review, 24% of them evaluated the relationship between salivary cortisol and hypercortisolism or CS [48, 49, 56, 65, 66, 69], 20% of the studies considered T2D [50, 51, 57-59], 20% evaluated stress or major depressive diseases [6, 48, 54, 55, 67], and finally 36% studied the relationship with periodontal disease [46, 52, 53, 60-64, 68].
Of the studies selected, 48% of them evaluated salivary cortisol levels using nmol/L, 20% used ng/mL, 4% assessed cortisol values using mmol/dL, 4% did not specify any unit of measure, 8% used pg/mL, and 16% applied µg/dL as a unit of measurement.
3.2. Risk of Bias
The Cochrane Collaboration tool was applied to assess the risk of bias for RCT in the articles included in this review (Table 3), using the judging criteria for risk of bias shown in Table S4. Criteria for judging the risk of bias in the ROBINS-I assessment tool are shown in Table S5. Bias analysis using the ROBINS-I-tool [70] for observational studies is shown in Table S6 (supplementary material). A moderate risk of bias was observed in this review.
Table (4a-d) shows the baseline characteristics of patients included in the selected studies.
Evidence of studies included in this systematic review (study design and aim, methods, results, and conclusions) is shown in Table S7 (supplementary material).
NHLBI quality assessment tool for controlled intervention studies is shown in Table S8 (supplementary materials. NHLBI quality assessment tool for observational cohort and cross-sectional studies is shown in Table S9.
4. DISCUSSION
Measurement of cortisol content in saliva provides an indirect assessment of overall health status [17]. In a healthy state, cortisol levels are tightly regulated to maintain homeostasis, as uncontrolled levels can be detrimental [71]. Studies have demonstrated that salivary cortisol measurement can be a simple and convenient screening tool for individuals with CS [72]. Similarly, in patients with type 2 diabetes, cortisol has been shown to promote gluconeogenesis and insulin secretion, contributing to insulin resistance [73]. In the context of stress and depressive disorders, lower salivary cortisol levels have been associated with unfavorable disease progression [74]. Furthermore, numerous studies have established a statistically significant relationship between periodontal disease indexes (such as CAL and PPD) and alterations in cortisol levels, indicating a correlation with disease severity [75, 76].
| Authors/Refs |
No. of Patients and % of Women |
Mean Age (years, SD or range) |
Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|---|
| Bäcklund et al., 2020 [48] |
Test: 22 (77%) Control: 155 (58.7%) | Test: NR Control: NR |
NR | HPA axis disease, glucocorticoid medication, diurnal rhythm issues, fever, oral problems |
| Lages et al., 2019 [49] |
Normal: 57 (61.4%) Suspected CS: 39 (64.1%) Proven CS: 31 (87.1%) |
Normal: 47.9 ± 11.09 Suspected: 51.54 ± 18.00 Proven: 46.19 ± 14.11 |
NR | Endocrine conditions, chronic pain, alcoholism, smoking, pregnancy, chronic diseases, psychiatric illness, shift work, certain medications |
| Garrahy et al., 2021 [56] |
23 (78.2%) | 35 (7-70) | Confirmed CS | Pseudo-Cushing’s, interfering medications |
| Ueland et al., 2021 [65] |
320 (54.3%) | 4-16 | NR | Glucocorticoids |
| Lin et al., 2019 [66] |
CS: 48 (81.2%) Control: 13 (76.9%) Normal: 21 (42.8%) |
CS: 43.1 ± 2.2 Control: 39.9 ± 5.5 Normal: 29.7 ± 0.8 | NR | NR |
| Mohamed et al., 2022 [69] |
54 (83.3%, 21 with CS) | 44.8 | High pre-test probability for CS | Cyclical, subclinical, pseudo-CS, non-compliant patients |
| Authors/Refs |
No. of Patients and % of Women |
Mean Age (years, SD or range) |
Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|---|
| Salehi et al., 2019 [50] |
T2D: 44, pre-diabetic: 44, healthy: 44 |
T2D: 54 ± 5.70 (40-60), pre-diabetic: 48.07 ± 8.68 (31-60), healthy: 42.86 ± 11.90 (30-60) | NR | BMI> 30, pregnancy, tobacco, drugs, alcohol, Addison’s disease, Cushing’s syndrome, thyroid disorders, recent injury/surgery, malignancy, corticosteroid/hormone therapy, mental/sleep disorders |
| Liu et al., 2005 [51] |
T2D: 141 (0%), no diabetes: 46 (0%) |
T2D: 61.8 ± 9.0, no diabetes: 58.7 ± 10.0 | Type 3 diabetes | Type 1 diabetes, glucocorticoids, dementia, acute illness, medications affecting cortisol, night-shift work, history of CS, autoimmune diabetes |
| Johar et al., 2016 [57] |
757 (48.8%) | 75 (65-90) | NR | Missing data on type 2 diabetes and covariates |
| Hackett et al., 2014 [58] |
3508 (24.9%) | 61.04 ± 5.94 | Consent to saliva samples, complete cortisol data within range | No saliva samples, cortisol values 3SD from mean, steroid medication, non-white |
| Hackett et al., 2016 [59] |
Normoglycemic: 2542 (26.8%), impaired fasting glucose: 518 (16.4%), diabetic: 210 (23.8%) |
Normoglycemic: 60.94 ± 5.90, impaired fasting glucose: 60.13 ± 5.72, diabetic: 61.45 ± 6.03 | Complete cortisol measures | Prevalent diabetes (at phase 7), incomplete cortisol sample |
| Authors/Refs |
No. of Patients and % of Women |
Mean Age (years, SD or range) |
Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|---|
| Mesa et al., 2014 [52] |
Cases: 41, 48.8% Controls: 36, 72.2% |
Cases: 53.7 ± 14.7 Controls: 37.8 ± 19.1 |
Cases: 4 teeth with PD ≥4 mm and AL ≥3 mm, bone loss. Controls: PD ≤3 mm and AL ≤2 mm | Cases: antibiotic/periodontal treatment in last 6/12 months, <5 teeth, corticoids/estrogens/immunosuppressants, systemic infection/neoplastic disease/pregnancy/breastfeeding/≥4 cups coffee/tea |
| Zhang et al., 2022 [46] |
Group 1: 200 Group 2: 200 Group 3: 200 50% of 600 |
20-50 | Group I: CP with PPD ≥5 mm, CAL ≥5 mm, bone loss, smoking ≥10 cig/day for 5 yrs. Group II: CP with PPD ≥5 mm, CAL ≥5 mm, bone loss. Group III: periodontally healthy, non-smokers. | Systemic disease/medications affecting cortisol, hyperpituitarism/pituitary/adrenal tumors, Cushing’s syndrome, high-dose corticosteroids, pregnancy |
| Bawankar et al., 2019 [53] |
Group 1: 25 Group 2: 25 Group 3: 25 46.7% of 75 |
30-65 | Group 1: periodontally healthy, non-smokers. Group 2: severe CP, PPD/CAL ≥5 mm, bone loss, current/history of smoking. Group 3: severe CP, PPD/CAL ≥5 mm, bone loss, non-smokers. | Psychiatric disorders, systemic disease, pregnancy, antibiotic/steroid/chemotherapy in last 6 weeks, acute illness, periodontal therapy in last 6 months |
| Fenol et al., 2017 [60] |
70 | 38.56 ± 10.878 | Minimum 20 teeth | Corticosteroids/immunosuppressants, Addison's disease/Cushing's syndrome, smoking, systemic conditions/psychiatric history, recent periodontal therapy |
| Obulareddy et al., 2018 [61] |
Group 1: 23, 47.8% Group 2: 23, 52.2% Group 3: 23, 60.9% Group 4: 23, 60.9% |
Group 1: 42.60 ± 7.32 Group 2: 40.95 ± 7.48 Group 3: 42.08 ± 8.97 Group 4: 45.78 ± 7.82 |
Age ≥30 years, >20 teeth, no systemic diseases/medications, no recent periodontal treatment. | Pregnant/lactating women |
| Naghsh et al., 2019 [62] |
Periodontitis: 45, 80% No periodontitis: 45, 88.9% |
Periodontitis: 37.1 ± 9.8 (23–55) No periodontitis: 4.8 ± 10.7 (20–55) |
At least 15 teeth, fasting before the test | Systemic conditions, corticosteroids/immunosuppressants, antibiotics in last 6 months, smoking/alcohol, pregnancy/lactation, acute illness |
| Refulio et al., 2013 [63] |
Periodontitis: 36 No periodontitis: 34 64.2% of 70 |
30-65 | Systemically healthy, non-smokers (30-65 yrs) | Corticosteroids/immunosuppressants, antibiotics in last 6 months, acute illness, <3 natural teeth, systemic conditions interfering with periodontal disease, recent antibiotics for periodontal treatment, smoking |
| Rahate et al., 2022 [64] |
Group 1: 30, 60% Group 2: 30, 46.7% Group 3: 30, 16.7% |
Group 1: 49.03 ± 10.43 Group 2: 51.93 ± 7.34 Group 3: 52.23 ± 7.14 |
Group I: periodontally healthy, non-smokers. Group II: stage III periodontitis, non-smokers. Group III: stage III periodontitis, smokers. | Psychiatric disorders, systemic disease, pregnancy, post-menopausal/lactating women, recent antibiotics/hormonal therapy, acute illness, immunosuppressive therapy, recent periodontal therapy |
| Develioglu et al., 2020 [68] |
Mild periodontitis: 26, 65.4% Moderate: 39, 51.3% Severe: 15, 53.3% |
Mild periodontitis: 38.23 ± 3.55 (35-49) Moderate: 42.66 ± 7.65 (35-62) Severe: 55.26 ± 6.94 (41-68) |
Not lactating, no prosthetic tooth restorations, no missing data in saliva samples/stress questionnaires | Diabetes, myocardial infarction, heart disease, allergies, smoking/alcohol, recent periodontal treatment/anti-inflammatory/antioxidant drugs, recent antibiotics |
| Authors/Refs |
No. of Patients and % of Women |
Mean Age (years, SD or range) |
Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|---|
| Pulopulos et al., 2020 [6] |
Low expectancy: 27, 100% High expectancy: 25, 100% |
Low expectancy: 20.52 High expectancy: 21.00 |
No use of medication affecting cortisol levels, cognitive and cardiovascular activity, no current (or history of) neurological or psychiatric illness, <10 cigarettes/day. | Outliers for cortisol indexes, missing data for baseline questionnaires and PASA, missing data for cortisol levels, subjects who performed similar stress tasks in a previous experiment |
| Vrshek-Schallhorn et al., 2013 [47] |
270, 72.2% | NR | NR | Corticosteroid medication, insufficient cortisol data, no follow-up interviews after baseline interview, current major depression, current or past post-traumatic stress disorder at baseline, dysthymic disorder, clinically significant psychotic symptoms, or bipolar disorder |
| Khan, 2020 [54] |
Non-depressive: 30, 53.33% Depressive: 30, 5.33% |
Non depressive: 35.73±6.89 (20-48) Depressive: 39.10±11.65 (18-70) |
Clinically confirmed by indicative criteria through the Diagnostic and Statistical Manual of Mental Disorders and Beck’s Inventory and acutely depressed patients satisfying the diagnostic criteria of Beck’s scoring. | Hyperaldosteronism, Cushing’s syndrome, and under treatment for exogenous steroid diseases |
| Khan et al., 2020 [55] |
Healthy: 30 Major depression: 30 |
18-60 | Depression | Cushing’s syndrome/disease, hyperaldosteronism pregnancy, antidepressants, or exogenous steroid drugs treatment |
| Yonekura et al., 2014 [67] |
63, 41.2% | 14.29 ± 0.51 | NR | NR |
4.1. Hypercortisolism and Cushing’s Syndrome
Hypercortisolism is a clinical condition characterized by prolonged exposure of tissues to steroid hormones, including cortisol. This condition is often associated with hypercortisolemia and, if prolonged, can lead to CS [5]. Cushing's syndrome can be triggered by prolonged systemic exposure to various glucocorticoids, whether they are exogenous or endogenous. Exogenous CS results from the iatrogenic use of glucocorticoids, while endogenous CS can be further classified as ACTH-related or ACTH-independent [77]. In cases where CS is suspected, the detection of late-night salivary cortisol levels can be a simple and minimally invasive screening procedure. This method has a sensitivity of 92% and a specificity of 96% and is often combined with the simultaneous evaluation of late-night salivary cortisone levels, providing optimal screening for patients with suspected undiagnosed CS [69, 78]. Salivary cortisol assessments have proven equally effective as plasma measurements and outperformed the measurement of glucocorticoid excretion in urine [79]. The evaluation of steroid levels using Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS) offers several advantages, including increased specificity and simultaneous quantification of multiple steroids.
The ratio of cortisone to cortisol in patients with CS is often associated with hypercortisolemia. Cortisone levels may be slightly but significantly more effective in diagnosing CS. However, results from patients undergoing the Dexamethasone Suppression Test (DST) showed a high level of diagnostic accuracy for both salivary glucocorticoids [48]. Several studies have assessed the efficacy of Late-night Salivary Cortisol (LNSC), but cut-off levels vary among different laboratories, ranging from 3.6 nmol/L to 15.2 nmol/L for adults [80]. For this reason, different studies have analyzed diverse populations, such as Portuguese or Chinese, to establish individualized cut-off values. The results have validated the diagnostic effectiveness of Midnight Salivary Cortisol (MSC), for example, in Chinese patients with Cushing's syndrome, consistent with findings from other nationalities. Hence, MSC is a precise biomarker useful for screening and clinical diagnosis [49, 66]. Salivary cortisol has reduced discriminatory capacity in the elderly and may be elevated in cases of hypertension or diabetes. Test ranges may need to be expanded for elderly subjects and patients with type 2 diabetes compared to younger, healthy individuals [51]. Children with CS require specific cut-off values for late-night salivary cortisol tests, with a value of around 2.4 nmol/L being used to distinguish between healthy children and those with CS. However, the lack of literature findings suggests that CS is an infrequent disease affecting young individuals [65].
Additionally, various factors should be considered when interpreting salivary cortisol levels. Tobacco use can increase salivary cortisol by inhibiting the expression of 11-β-hydroxysteroid Dehydrogenase type 2 (11 β-HSD2). Therefore, smoking should be avoided on the day of sample collection [26, 80]. Repeated measurement of LNSC over several consecutive nights allows for the establishment of a secretory pattern of cortisol secretion. This can be helpful in cases of cyclical CS, a rare condition characterized by alternating periods of hypercortisolism and periods of normal cortisol levels when clinical signs and symptoms of CS decrease [81]. Screening tests should be performed more than once, particularly in cases of pre-test clinical uncertainty. In such circumstances, patients may need to undergo re-evaluation over 6-12 months [56]. Incorrect timing of sample collection and other variables, such as specimen contamination with blood and differences in collection protocols (e.g., number and frequency of samples), can contribute to variability in the quantification of late-night salivary cortisol and late-night salivary cortisone [82].
4.2. Pre-diabetic and Type 2 Diabetes (T2D) Conditions
Shifting the focus to the association between Salivary Cortisol (SaC) and prediabetic or diabetic conditions, there appears to be a relationship between an increase in SaC and glycemia. Studies have demonstrated that diabetic patients have higher SaC levels than pre-diabetic individuals, and pre-diabetic patients have higher SaC values than healthy individuals [50]. When investigating secretory cortisol patterns, it has been found that both the Cortisol Awakening Response (CAR) and Late-night Salivary Cortisol (LNSC) are increased in individuals with type 2 diabetes. Higher values of Hemoglobin A1c (HbA1c), a marker of long-term glucose control, have also been linked to diurnal and evening cortisol values [57, 83]. Moreover, oral conditions associated with diabetes, such as dry mouth and higher glucose levels in crevicular fluid, can promote the development of dental caries, resulting in an increased mean number of Decayed, Missing, and Filled permanent Teeth (DMFT) index [50]. Elevated salivary cortisol levels appear to elevate the susceptibility to dental caries. While the precise linkage remains unclear, heightened cortisol levels could potentially heighten the risk of dental caries by augmenting glucose concentrations in the gingival crevicular fluid, particularly in individuals with diabetes or pre-diabetes [50]. Patients with type 2 diabetes have been shown to have flatter diurnal cortisol secretory patterns (lower cortisol levels during the day and higher levels in the evening) compared to non-diabetic individuals. Increased LNSC levels (≥4.3 nmol/L) may indicate a clinical suspicion of CS, but are not necessarily associated with elevated Body Mass Index (BMI), which is commonly associated with the disorder. High LNSC values were found in individuals with diabetes but without CS [51, 58, 78]. A flattened diurnal cortisol secretory pattern has been identified as a predictive parameter for the subsequent onset of prediabetes or type 2 diabetes, along with increased LNSC levels. This suggests that evening cortisol levels serve as an early warning sign in initially non-clinical individuals [59]. Stress may be the missing puzzle piece that links the flattened diurnal cortisol slope and increased night-time cortisol values. Many studies have pointed to stress and other related factors as contributors to an increased risk of developing type 2 diabetes [84]. Subjects diagnosed with diabetes demonstrated a circadian cortisol pattern marked by diminished morning levels and increased cortisol concentrations in the afternoon and evening [8, 85]. The Hypothalamic-pituitary-adrenal (HPA) axis is activated by stress, leading to cortisol secretion. Acute and chronic stress factors have been associated with alterations in diurnal and nocturnal cortisol patterns [86].
4.3. Stress and Depression
As previously mentioned, salivary cortisol serves as a valuable biomarker in the diagnosis of stress conditions and depression [87]. The Cortisol Awakening Response (CAR) and major life events have been found to independently predict the occurrence of major depressive disorders. CAR notably predicts the onset of depression and the recurrence of depressive episodes, with the latter parameter exhibiting stronger predictive power [47]. When comparing individuals with severe depression to non-depressed individuals, it is evident that the former group has elevated salivary cortisol levels. This association may also be linked to a family history of depression and other notable health characteristics [54]. Salivary cortisol has also shown utility in tracking disease progression [88]. Stress-related disorders can arise from an exaggerated response of the Hypothalamic-pituitary-adrenal (HPA) axis to stressors. The heightened sensitivity to stressful events is influenced by prolonged negative expectations regarding future stressors [6]. Individuals with elevated expectations actively foresee stressful situations, culminating in a prolonged anticipatory response of the dorsolateral prefrontal cortex and the suppression of the amygdala. This attenuation of the amygdala's reaction to stress consequently leads to diminished activation of the Hypothalamus-pituitary-adrenal (HPA) axis [6]. Efforts to reduce stress reactivity have shown promise in mitigating negative health consequences associated with stress-related factors [89]. In addition to salivary cortisol, depression has also been found to be associated with Body Mass Index (BMI). Recognizing this relationship can aid in the identification of individuals in the pre-clinical stage, and early intervention and treatment can improve clinical outcomes [55]. Elevated cortisol levels among individuals with depression suggest a potential link between these levels and the predictive value of the HPA axis in disease onset and prognosis. Addressing the risk factors associated with depression's emergence holds paramount importance. Implementing counseling and advocating for lifestyle adjustments among the general populace could contribute to BMI enhancement. Prolonged and excessive cortisol secretion contributes to metabolic disturbances, increasing susceptibility to obesity and exacerbating depressive symptoms in patients [54]. In adolescent subjects, the analysis of salivary cortisol twice daily (in the morning and at night time) for three consecutive days is indicative of their depressive states. Single measurements may not be sufficient to distinguish between healthy individuals and those with depression [67].
4.4. Periodontal Disease
In the current review, nine out of the 25 studies examined the relationship between salivary cortisol and periodontitis. Patients with periodontitis exhibited concurrent clinical signs of the disease, such as a higher Plaque Index (PI), gingival inflammation/Bleeding on Probing (BOP), and tooth loss. The underlying hypothesis for these findings may be related to more frequent risky behaviors, poor oral hygiene, and increased tobacco consumption [52, 90]. Elevated levels of salivary cortisol have been associated with greater Probing Pocket Depths (PPD) in patients with periodontitis (ranging from 5-7 mm) [60]. When contrasting healthy individuals with those diagnosed with Chronic Periodontitis (CP), healthy subjects exhibited minimal cortisol levels. This observation implies that within a healthy oral setting, cortisol might contribute to facilitating controlled chemotaxis and governing immune and inflammatory reactions [61, 91].
In addition to the correlation between Salivary Cortisol Levels (SCL) and PPD, recent literature highlights other significant salivary biomarkers associated with periodontitis. Matrix Metalloproteinase-8 (MMP-8) has been widely studied, with elevated salivary levels consistently linked to periodontal tissue destruction and inflammation. This enzyme, involved in collagen degradation, serves as a reliable marker for detecting periodontal disease severity [92]. Another noteworthy biomarker is Interleukin-1β (IL-1β), a pro-inflammatory cytokine found in higher concentrations in periodontitis patients, correlating with the extent of periodontal inflammation [93]. Similarly, C-reactive Protein (CRP) in saliva, reflecting systemic inflammation, has shown elevated levels in periodontitis patients, indicating its potential as a marker for disease risk and progression [94].
The literature highlights Peptidoglycan Recognition Protein 1 (PGLYRP1) as a relevant biomarker. Elevated PGLYRP1 levels in saliva are associated with periodontal inflammation, suggesting its role in the immune response to bacterial infection. Similarly, Macrophage Inflammatory Protein-1α (MIP-1α) is a significant biomarker, with higher salivary levels indicating the recruitment of inflammatory cells to periodontal tissues. Recent studies have further explored the association between PGLYRP1 and periodontal inflammation. For example, research has shown that salivary PGLYRP1 levels correlate with gingival inflammation and the presence of caries, indicating its potential as an early diagnostic marker for periodontal disease [95]. Another study examined the impact of peri-implant treatment on salivary PGLYRP1 levels, showing a significant reduction post-treatment, suggesting that PGLYRP1 could be valuable in monitoring treatment response [96].
Regarding MIP-1α, research indicates that salivary concentrations of this protein are elevated in patients with periodontitis, reflecting disease severity and response to treatment [97]. Furthermore, MIP-1α plays a crucial role in recruiting inflammatory cells to inflammation sites and bone resorption areas, highlighting its importance in the pathogenesis of periodontal diseases [98].
These studies support the use of PGLYRP1 and MIP-1α as salivary biomarkers for diagnosing and monitoring periodontal diseases, providing non-invasive tools to assess inflammation and treatment response. In addition to these biomarkers, there has been found a significant association between SCL and responses to Spielberger State-Trait Anxiety Inventory (STAI) questionnaires [62]. When comparing STAI questionnaire scores between CP patients and gingivitis patients, higher scores were observed in the CP group [68]. It is plausible to suggest that the correlation between cortisol and CP could stem from the suppressive influence of the HPA axis on the inflammatory response, given that cortisol inhibits all aspects of the immune reaction [63]. Cortisol's effect includes a reduction in the migration of white blood cells to the inflamed region and a decrease in the phagocytosis of impaired cells [60]. Cortisol levels are closely related to the severity of periodontal disease, and in smokers, salivary cortisol levels may be higher, leading to increased tissue destruction, which reflects an explicit, but temporary impact on the neuroendocrine system [64].
However, smoking presents itself as a potential confounder. Extensive scrutiny of the literature indicated a consensus on the adverse influence of smoking on both the incidence and progression of periodontitis. Therefore, the salivary cortisol levels observed in periodontal patients who smoke may be attributable to a cumulative effect [53, 64]. Moreover, it has been shown that higher levels of cortisol are strictly related to the presence of Porphyromonas gingivalis in subjects affected by chronic periodontitis [99]. The diagnosis, staging, and clinical management of CP should consider both physical and psychological aspects, and salivary biomarkers, particularly cortisol, MMP-8, IL-1β, CRP, PGLYRP1, and MIP-1α, may serve as useful indicators in identifying underlying physiological factors associated with periodontal disease and smoking.
Salivary cortisol is a reliable parameter (p<0.0001) compared to serum cortisol (p > 0.05), as it reflects the biologically active unbound cortisol in serum and is not affected by variations in the concentration of cortisol-binding globulin, which can alter cortisol level readings [53]. It should be noted that other conditions that have not been investigated extensively, such as rheumatoid arthritis or lichen planus, may also be related to alterations in salivary cortisol. Indeed, given that around 50% of OLP patients experience sleep disturbances, anxiety, and depression, it becomes imperative to evaluate the psychological well-being of all individuals affected by this condition, but more in-depth studies are needed [100, 101].
4.5. Limitations of this Study and Future Perspective
Acknowledging certain limitations within this report is crucial. The electronic search process lacked the involvement of information specialists or academic librarians, potentially affecting search comprehensiveness. Moreover, the employed search criteria might have been overly specific for the intended scoping question [102]. Additionally, challenges may have arisen in comparing findings due to potential variations in samples, driven by the inherent variability of salivary cortisol influenced by individual factors and diurnal secretory patterns. Indeed, cortisol values can be up to 10 times higher in the morning if compared to nighttime [64] Variability in salivary test characteristics and parameters, stemming from diverse laboratory practices and protocols, may have complicated result comparisons. The wide array of salivary sampling devices in the current market may have added another layer of heterogeneity, potentially influencing test outcomes and subsequent clinical trial conclusions.
To address these limitations and advance our understanding, future investigations should explore the correlation between various conditions and their potential impact on salivary cortisol levels. Establishing definitive and standardized cut-off values for salivary cortisol testing is essential to enhance the reliability and clinical utility of these measures.
Additionally, other conditions should be analyzed more in-depth, as only a limited number of studies have explored the involvement of the HPA axis in individuals with endometriosis. In endometriosis, a chronic inflammatory state and heightened cardiovascular risk are characteristic features akin to some other gynecological conditions, like PCOS, which are linked to relative hyperaldosteronism. Preliminary findings indicate a potential association between elevated biological aldosterone activity and endometriosis. Reduced cortisol levels may also play a significant role in this condition, particularly in its early stages. However, further research is required to further investigate this novel aspect of endometriosis pathogenesis.
CONCLUSION
Salivary cortisol is a key biomarker for diagnosing and managing various conditions, like Cushing's disease, diabetes, stress, depression, and periodontal disease. Its simplicity and reliability offer insights into the body's HPA axis, with standardized testing potentially improving precision. Ongoing research aims to uncover connections between these conditions, shaping future diagnostic and treatment approaches. This review encourages further exploration into salivary cortisol's role across different health issues to refine healthcare strategies.
AUTHORS’ CONTRIBUTION
F.S., A.B.G., and A.S.: Contributed to conceptualization, methodology, visualization, supervision, and project administration; M.P. and A.S.: Curated the software; A.S. and F.S.: Contributed to validation, formal analysis, resource allocation, and data curation; F.P. and F.S.: Performed investigation; M.G. and M.P.: Prepared the original draft; M.G., M.P., and A.S.: Conducted writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| CS | = Cushing's Syndrome |
| HPA | = Hypothalamic-Pituitary-Adrenal |
| CP | = Chronic Periodontitis |
| TNF-α | = Tumor Necrosis Factor alpha |
| CAR | = Cortisol Awakening Response |
AVAILABILITY OF DATA AND MATERIAL
The data sets used and/or analysed during this study are available from the corresponding author [A.S] upon request.
SUPPLEMENTARY MATERIALS
Table S1: PRISMA 2020 checklist. Table S2: Search strategies for electronic databases. Table S3: Summary table of studies excluded in this systematic review. Table S4: Criteria for judging risk of bias using the “risk of bias” assessment tool. Table S5: Criteria for judging the risk of bias in the ROBINS-I assessment tool. Table S6: Bias analysis using the ROBINS-I tool for observational studies. Table S7: Evidence of studies included in this systematic review. Table S8: NHLBI quality assessment tool for controlled intervention studies. Table S9: NHLBI quality assessment tool for observational cohort and cross-sectional studies.




