All published articles of this journal are available on ScienceDirect.
The Efficacy of Leukocyte-Platelet-Rich Fibrin in Combination with Sub-Epithelial Connective Tissue Graft in Peri-Implant Soft Tissue Augmentation: A Randomized Controlled Clinical Trial
Abstract
Aims and Background
The aim of the current study is to assess and compare the efficacy of the leukocyte-platelet-rich fibrin (L-PRF) in combination with sub-epithelial connective tissue graft (SCTG) and SCTG only in promoting aesthetic results and strengthening the state of the soft tissue surrounding implants.
Materials and Methods
A parallel-arm randomized controlled clinical trial was used for this investigation. A total of 120 patients who had missing teeth with a thin gingival biotype [less than 1.5 mm] were included in this study. The patients of Group I were treated with PRF and SCTG during second-stage implant surgery, while the patients of Group II were treated with SCTG only. Treatment outcomes included the assessment of the width and thickness of the keratinized tissue at the baseline, 3 and 6 months postoperatively; pro- and anti-inflammatory cytokines (IL-1β, TNF-α, and IL-4) in the peri-implant crevicular fluid on the 1st, 7th and 30th days after surgical procedure; swabs from the surface of postoperative wounds of the mucous membrane on days 3, 5, 7, and 10; Pink aesthetic score (PES) 6 months after prosthesis placement; and laser Doppler flowmetry on days 1, 7, and 14 after augmentation.
Results and Discussion
Patients who received L-PRF+SCTG had a significant increase in keratinized tissue thickness (KTT) (p = 0.08) than those who received SCTG only (KTT 1.86 ± 0.17 Vs 1.48 ± 0.15) 6 months after surgery. The mean (±SD) of PES was found to be 13.1 (±0.02) for Group I and 11.3 (±0.08) for Group II. The cytology, LDF, and local immunoassay analysis demonstrated faster epithelialization and better revascularization in Group I.
Conclusion
The use of L-PRF and SCTG is an effective method in augmenting peri-implant soft tissue and improving gingival biotype and aesthetic outcomes, which would help overcome complications and increase patients’ satisfaction.
1. INTRODUCTION
Dental implants are a common method of treating lost teeth [1]. According to literature data, the thickness and width of keratinized tissues determine the viability of implants over time, their stability in function, and aesthetics [2].
It has been proven that bone loss around implants occurs with a vertical tissue volume of less than 2 mm [3]. Moreover, a width of keratinized tissues of less than 1.5 mm can lead to soft tissue recessions, plaque accumulation, and the formation of pathological pockets [4]. Thick gingival tissue [greater than 2.5 mm], according to Abrahamsson et al., can greatly stop crestal bone loss surrounding implants [5]. Thus, peri-implant soft tissue augmentation leads to a favorable treatment prognosis. Soft tissue management around dental implants may be accomplished prior to the surgical phase, after the surgical phase, before loading, or after loading. Kadkhodazadeh et al. [3] reported that it is possible to use soft tissue grafts immediately or during the second stage of implant surgery in case of inadequate keratinized soft tissues and sufficient bone level.
The use of soft tissue grafts has become a substantial element in implant surgery [6]. Elkashty et al. [7] reported that the aesthetic and functional outcomes were much higher when using subepithelial connective tissue graft (SCTG) for peri-implant soft tissue augmentation. Moreover, SCTG could promote epithelial proliferation [8].
An autologous membrane using platelet-rich fibrin (PRF) is an innovative technique to promote wound healing [9]. Numerous investigations have demonstrated that PRF was utilized to enhance the gingival phenotype [10-12]. The components of PRF include fibrin, platelets, cytokines, and several growth factors, including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-beta1 (TGF-1), which serve as a scaffold and encourage the migration and proliferation of epithelial cells [13, 14]. PRF is also known to have an anti-inflammatory effect and improve gingival perfusion; thus, it has been used in periodontal regenerative procedures [15-19].
Several protocols have been described in studies for PRF preparation [20-22]. The most recent of these protocols is the advanced platelet-rich fibrin (A-PRF) method with the “Low-speed centrifugation concept”, which shows a high number of platelets and leukocytes, as well as the higher and sustained release of growth factors, compared with leukocyte-platelet-rich fibrin L-PRF [23, 24]. On the other hand, some recent investigations showed better characteristics of L-PRF [25]. Moreover, several studies proved the positive impact of L-PRF on the fibroblasts involved during wound healing [26].
Even though applying SCTG or PRF membrane has been shown to be effective in enhancing the soft tissue surrounding implants, further research is necessary to fully understand the utilization of PRF in conjunction with SCTG.
Different methods have been introduced to assess gingival thickness, epithelialization, local immunity, gingival perfusion, and aesthetic outcomes. The trans- gingival method of measurement is commonly used to assess mucous thickness. The thickness of facial gingiva is commonly measured after anesthesia by piercing the keratinized gingiva with the periodontal probe or another sharp instrument [27, 28]. For this purpose, the use of cone-beam computed tomography (CBCT) with a labial retractor during the examination has been recommended by several authors [29, 30].
The rate of epithelialization can be evaluated by cytology analyses, which helps investigate epithelial cell differentiation and the influence of PRF on wound healing [31].
Several studies have demonstrated how dental implant abutments could lead to inflammation and the elevation of pro-inflammatory mediators that can be quantified under conditions associated with localized inflammation [32, 33]. Determination of cytokines in gingival crevicular fluid is widely used to estimate the results of surgery on periodontal tissues [34, 35]. To understand local immunity after soft tissue surgery using PRF, it is reasonable to study the amount and ratio of pro-inflammatory and anti-inflammatory cytokines in the peri-implant crevicular fluid. Interleukin IL-1ß was the most studied cytokine, followed by tumor necrosis factor TNF-α. Peri-implant crevicular fluid [PICF] containing inflammatory mediators can be used as a criterion for the diagnosis of postoperative complications and peri-implant infection [36].
The study of gingival blood flow can also be one of the criteria for assessing soft tissue healing, especially after the use of grafts in periodontal surgery and dental implantation. A noninvasive method of measuring capillary blood perfusion [blood flow, volume, and velocity] is the laser Doppler flowmetry (LDF) [37].
Evaluation of aesthetic outcomes is one of the crucial components in assessing the results of surgical procedures. Aesthetics in implant treatment depends on various factors: proper implant position, adequate bone on the buccal surface, shape, form of the final crown, and peri-implant soft tissue status [38].
The condition of peri-implant soft tissues is very important for patients. The use of reliable indices is an objective clinical aid to monitor the results over time. Various indices have been proposed to evaluate aesthetic outcomes, such as the Peri-Implant and Crown Index, Implant Crown Aesthetic Index, Pink Esthetic Score, and White Esthetic Score. The Pink Esthetic Score (PES) could be a useful tool for monitoring long-term soft tissue alterations [39].
Other studies have not performed extensive evaluations, including assessments of keratinized gingival thickness and width, local immunity, laser Doppler flow- metry, and post-prosthetic aesthetic evaluations after SCTG and PRF augmentation.
The aim of the current study is to compare the efficacy of the L-PRF in combination with SCTG and SCTG only in increasing the state of the soft tissue surrounding implants and improving their appearance. The comparison is provided by measurement of the width and thickness of keratinized tissue and by assessment of the PES, LDF, cytological analysis, and evaluation of pro-inflammatory and anti-inflammatory cytokines in PICF.
2. MATERIALS AND METHODS
The current study is a prospective, interventional, single-blinded, randomized parallel trial conducted in Almaty, Kazakhstan. The study protocol was approved by the Research Ethics Committee of the Asfendiyarov Kazakh National Medical University (Approval number: Nº6[112] from May 26, 2021). The trial was registered on www.isrctn.com (registration number ISRCTN27670172) and was prepared based on the CONSORT guidelines for reporting randomized controlled trials.
The primary outcome involves the assessment of the effectiveness of combining L-PRF with SCTG versus SCTG alone in enhancing peri-implant soft tissue and aesthetic outcomes.
Selection of Participants: About 130 patients aged between 18 and 60 years with single or multiple non-restorable teeth were selected according to inclusion and exclusion criteria. The inclusion and exclusion criteria are shown in Table 1.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age: 18-60 | Pregnancy |
| Patients with single or multiple non-restorable teeth | Systemic diseases |
| Thin biotype of gingiva | Thick biotype of gingiva |
| Inadequate keratinized tissue [width less than mm] | - |
| Sufficient bone | Insufficient bone |
| Patient consent approval and signing | - |
| Absence of untreated periodontal disease | - |
Sample size determination: Sample size calculation was achieved using Power and Sample Size Calculation Software [Version 3.1.2, W.D. Dupont and W.D. Plummer, USA] to ensure the study had enough power to detect a significant difference between the groups if one existed. The equation used for sample size calculation is as follows:
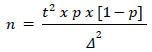 |
Where:
- n is the sample size;
- t is a constant [of the distribution of sample means];
- Δ is the maximum permissible error for this study;
- p is the proportion of the studied parameter.
When calculating the sample size, we allowed for the maximum standard deviation that is possible with the relative proportion of the studied parameter [6%]. The significance level was 5%, with a confidence level of 95% (t = 1.96). In this case, the minimum sample size for the two groups (test and comparison) should have been at least n = 87 patients.
Sample distribution: The patients were randomly assigned to Groups I and II using a random number method. In Group I [the test group], patients received delayed dental implantation combined with SCTG and L-PRF membrane. In Group II [the comparison group], patients underwent delayed dental implantation with SCTG only. Group III [the control group] consisted of 20 patients with a thin biotype of gingiva who underwent standard dental implantation without any additional surgery that could lead to the thickening of the mucous membrane. The patients for this control group were selected specifically for their thin gingival biotype, and their assignment to Group III was based on the absence of any augmentation procedures during implantation. The recruitment for Group III was focused on ensuring a natural control group to compare with the intervention groups, helping to isolate the effects of the additional procedures used in Groups I and II. The CONSORT flowchart, which investigated the procedure of enrollment, allocation, follow-up, and analyses, is presented in Fig. (1).
The information provided meets ethical standards, including the requirement for participants to provide written informed consent before inclusion in the study. It is also in line with key guidelines for medical research involving human subjects, such as the Declaration of Helsinki and the European Medicines Agency Guidelines for Good Clinical Practice. Furthermore, the approach to keep personal information provided by participants separate and modified from the data, as well as maintaining blinding for outcome assessors and data analysts, aligns with best practices for protecting the privacy and confidentiality of research participants. This approach ensures accuracy and impartiality in the data analysis without compromising the privacy of the individuals involved.
The dental implant procedure involved the harvesting of a subepithelial connective tissue graft (SCTG) from the lateral part of the hard palate during the second-stage implant surgery. This process included making two horizontal and two vertical incisions perpendicular to the mucosal surface, each 1.0–1.5 mm deep, according to Bosco et al. [40] technique. The mucosal defect on the donor site was closed with a collagen membrane and sutured. The SCTG graft was then positioned on sterile gauze, moistened with a saline solution, and de-epithelialized with a scalpel blade (Fig. 2). Subsequently, the SCTG was placed inside the vestibular pouch using a mattress suture to secure it in place.
In Group I, the L-PRF membrane was prepared using a blood sample obtained from a vein, typically 10 ml, and transferred to a free anticoagulant tube. The blood sample was then centrifuged at 700 g for 8 minutes in centrifuge (Hettich EBA 200, Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany) and the resulting fibrin clot was compressed in the PRF box (HBXPRF, Beijing Hanbaihan Medical Devices Co., Ltd., Beijing, China) to obtain the L-PRF membrane, which was then applied over the subepithelial connective tissue graft. L-PRF preparation protocol was performed following the methods of Miron et al [41]. During the surgical procedure, the flap was formed, and the initial keratinized tissues were shifted to the lingual side. The graft and PRF membrane were stabilized with an absorbable suture (Vicryl 5-0, Johnson & Johnson MedTech, New Brunswick, New Jersey) (Fig. 3).
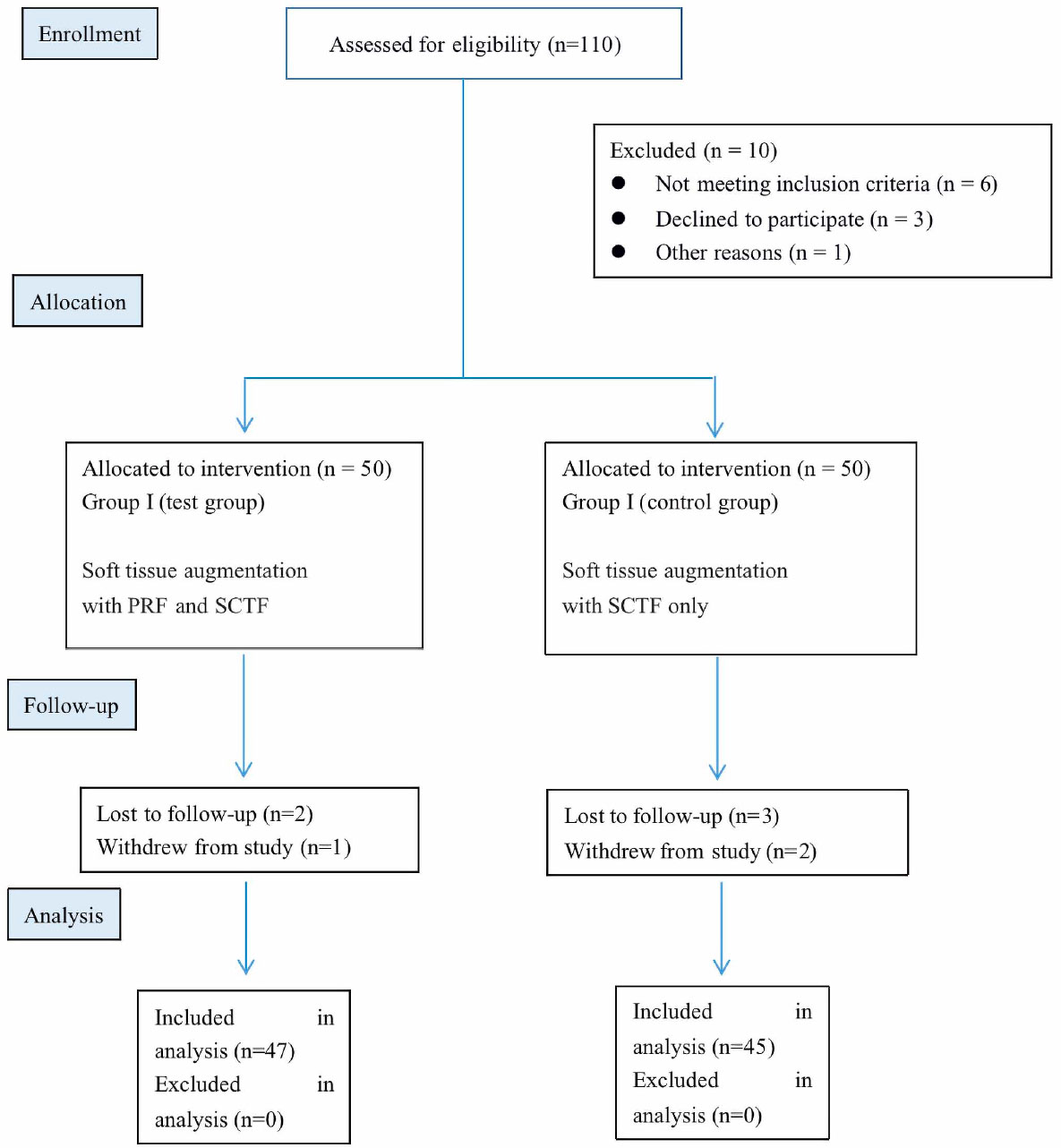
Flowchart of the participants (consort flowchart).
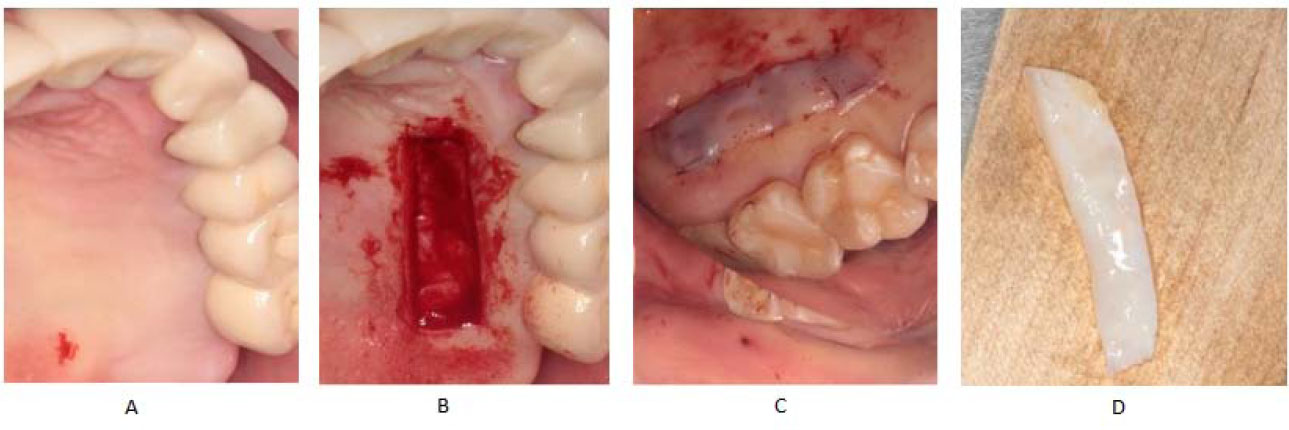
Harvesting of the SCTG.
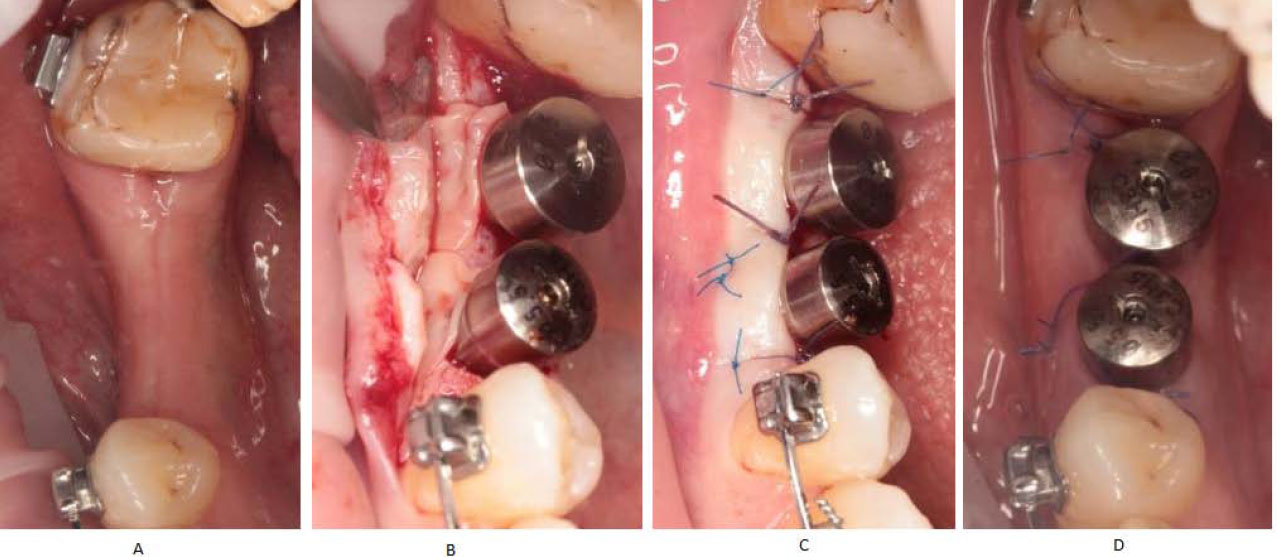
Group I case. L-PRF membrane with SCTG during 2nd stage of delayed dental implantation.
The patients were prescribed antibiotics and anti-inflammatory medication for five days following the operation. Additionally, patients were advised to refrain from brushing the surgical sites and instructed to conduct mouth rinses with Chlorhexidine for 14 days. Sutures were removed after 10 days. Furthermore, the patients were provided with a follow-up schedule at 3 and 6 months postoperatively.
2.1. Assessment of Width and Thickness of Keratinized Tissue
After enrollment, the following clinical parameters were assessed at baseline and at three and six months after the surgical procedure. Using a graded periodontal probe, the width of the keratinized tissue was evaluated by measuring the distance between the mucogingival junction (MGJ) and free gingiva, according to Rijal et al. [42]. Thickness was measured using cone-beam computed tomography (CBCT) with Ez3D-1 software (Vatech, Hwaseong-si, Gyeonggi-do, Korea) before and at 3 and 6 months after the surgical procedure as suggested by Schwarz et al. [43]. The measurement of the mucosal thickness for the determination of the biotype involved placing dental cotton swabs in the vestibule site of the oral cavity. Measurements were then carried out in the frontal and sagittal dimensions in areas corresponding to the vestibular cortical plates of the root of the examined tooth, in the projection of the central axis of the tooth, from the top of the cortical plate to the mucogingival junction (Fig. 4).
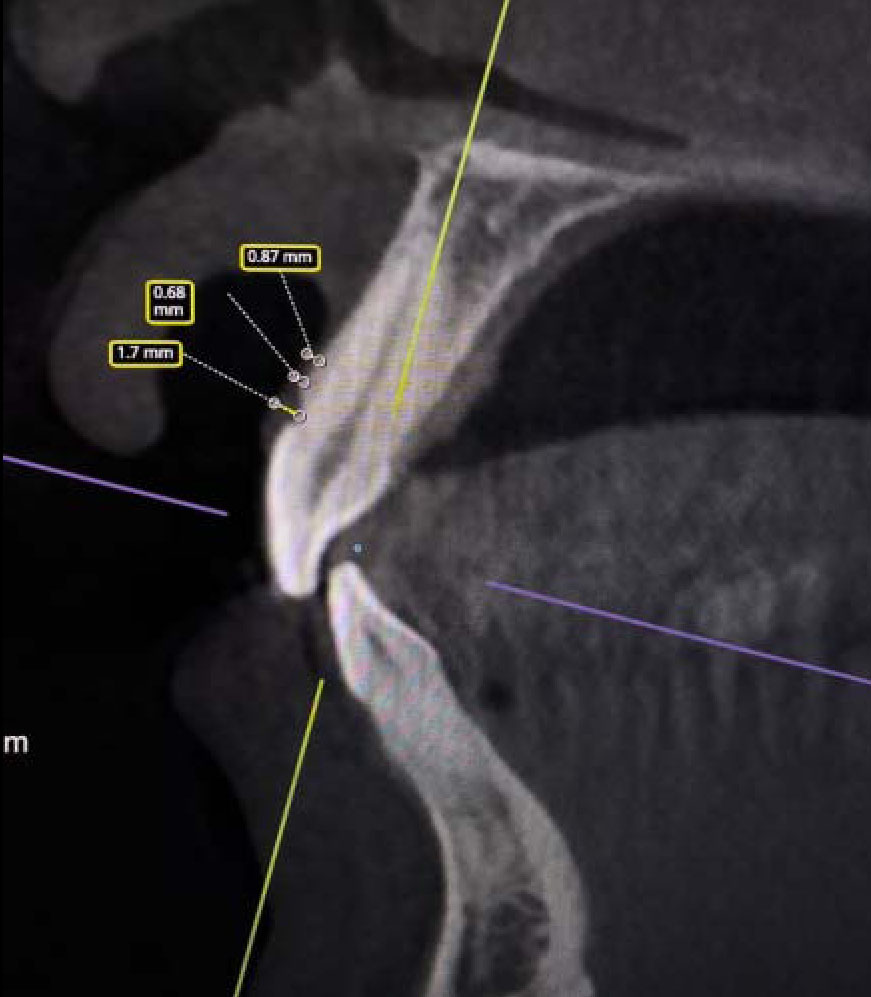
Principe of measuring of the thickness of keratinized mucosa on CBCT.
2.2. Cytological Assessment
The materials for the study consisted of swabs collected from the surface of postoperative wounds of the mucous membrane on days 3, 5, 7, and 10. The swabs were obtained using a sterile metal spatula and transferred to adhesive glass slides to prepare thin smears. The obtained smears were then dried and fixed in a mixture of ethyl alcohol-acetone solutions (in a weight or mass ratio of 1:1) for 5 minutes and stained with methylene blue according to May-Grunwald (15 min) and eosin azure according to Romanovsky-Giemsa (30 min). Photographs of the cytogram cells were captured using a Leica morphodensitometric system (Leica Microsystems GmbH, Wetzlar, Germany), including a DM 1000 microscope and a DFC-320 digital camera. This setup was used to obtain images of cytogram cells in jpeg format. For the convenience of counting the cytogram of the epithelium of the oral mucosa, 6 stages of epitheliocyte differentiation were identified by cytological parameters. The cytological analysis was performed following the method of Nanayakkara et al. [44].
2.3. Local Immunity Assessment
The evaluation of treatment outcomes included assessing the concentration of pro- and anti-inflammatory cytokines (specifically IL-1β, TNFα, and IL-4) in the peri-implant cervicular fluid on the 1st, 7th, and 30th days after the operation. This was performed using a sandwich enzyme-linked immunosorbent assay (ELISA) kit (Vektor-Best, Russia) in accordance with the manufacturer’s instructions. The peri-implant cervical fluid collection protocol was previously described by Tomás et al. [45]. According to this protocol, sterile pins were placed between the edges of the sutured wound for the evaluation of cytokines. The pins were then transferred to 1.0 cc of sterile saline in an Eppendorf tube, where fluid samples were collected between 8 and 10 a.m. Participants were instructed to refrain from eating, chewing, and drinking for at least one hour before collection. The detection range of the ELISA kit was from a minimum of 5 pg to a maximum of 200 pg/ml, and the results were expressed in pg/ml.
2.4. Gingival Blood Flow Assessment
Laser Doppler flowmetry (LDF) measurements were conducted after 1, 7, and 14 days to evaluate healing and wound evolution. For the LDF measurement, the probe was positioned 4 mm above the cervical line and distanced using a gingival dam. A silicone rubber holder was used to secure the LDF probe (Fig. 5). The technique outlined by Carmen et al. [46] was used to measure gingival blood flow.
2.5. Pink Esthetic Score Assessment
The Pink Esthetic Score (PES), introduced by Belser et al. [47], was measured 6 months after the prosthetic phase. The soft tissue level, soft tissue contour, soft tissue color, and texture, mesial and distal papilla, and alveolar process deficits were the seven factors that were assessed. A maximum score of 14 was possible using a scoring system with 0 being the lowest value and 2 being the highest.
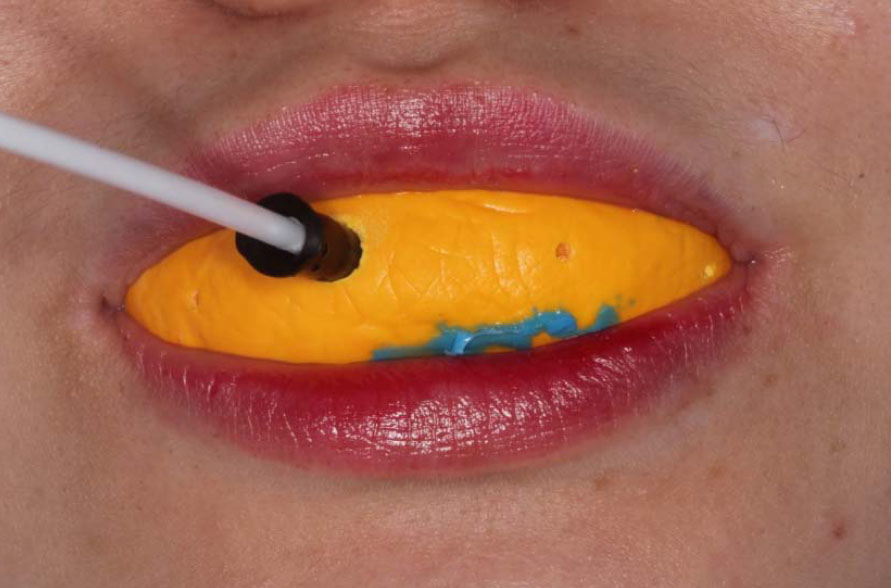
Measurement of LDF.
2.6. Statistical Analysis
Statistical processing of the obtained data was conducted using the SPSS program (IBM Corp., Version 21.0, Armonk, NY). Mean values were determined and expressed as the arithmetic mean (M), standard deviation (SD), median (Me), 25th and 75th percentiles (P25, P75). The distribution of all parameters was tested using the Shapiro-Wilk test, which revealed that the data did not follow a normal distribution in all cases. As a result, to ascertain the significance of differences, the Mann–Whitney U-test was utilized for inter-group comparison with a significance level set at p<0.05. The Kruskal-Wallis H-test was employed to determine whether there were statistically significant differences in the outcomes among the three groups. No parametric tests were used because the data distribution did not meet the assumptions of normality.
3. RESULTS
A total of 112 patients in the period between the years 2021 and 2022 were included in this study, with an age range from 18 to 60 years [61 females and 51 males] (Table 2).
3.1. Width and Thickness of Keratinized Tissue Analysis
In regard to the peri-implant soft tissue phenotype, a significant increase in facial tissue thickness was observed with both treatment options at 3 and 6 months post-surgery compared with baseline. Specifically, the comparison 3 and 6 months after the surgical procedure revealed a significantly higher enhancement of keratinized tissue thickness in patients of Group I with PRF+SCTG (1.88 mm ±0.14, 1.86 mm ±0.17) compared to patients of Group II with SCTG only (1.52 mm±0.13, 1.48 mm ±0.15); the difference was statistically significant with a p-value of < 0.1 (Table 3). However, the statistical significance of the difference in the width of keratinized tissues between Group I and Group II was not detected.
| - | Test Group PRF+SCTG [n = 47] |
Comparison Group SCTG [n = 45] |
Control Group [n = 20] |
p-value |
|---|---|---|---|---|
| Age/years | 55 ± 5.7 | 54 ± 5.2 | 55 ± 5.1 | N/A |
| Average thickness of facial gingiva before dental implantation | 0.93 ± 0.12 | 0.92 ± 0.07 | 0.92 ± 0.08 | ˃ 0.5 |
| Average width of keratinized tissue before dental implantation | 6.75±1.65 | 6.65±1.65 | 6.50±2.17 | ˃ 0.5 |
| - | Baseline | 3 Months after Surgical Procedure | 6 Months after Surgical Procedure | |
|---|---|---|---|---|
| Average thickness of facial gingiva [KTT] | ||||
| Group I PRF+SCTG [n = 47] |
0.93 ± 0.12 | 1.88 ± 0.14 | 1.86± 0.17 | |
| Group II SCTG [n = 45] |
0.92 ± 0.07 | 1.52 ± 0.13 | 1.48 ± 0.15 | |
| Group III control [n = 20] |
0.92 ± 0.08 | 0.83 ± 0.12 | 0.78 ± 0.14 | |
| p value | U test | ˃ 0.5 | = 0.096 | = 0.08 |
| H test | = 0.6 | = 0.073 | = 0.07 | |
| Average width of keratinized tissue [KTW] | ||||
| Test group PRF+SCTG [n = 47] |
6.75±1.65 | 7.28±1.12 | 7.13±1.34 | |
| Comparison group SCTG [n = 45] |
6.65±1.65 | 7.17±1.33 | 7.11±1.28 | |
| Group III control [n = 20] |
6.50±2.17 | 6.02±1.11 | 5.3±1.32 | |
| p value | U test | ˃ 0.5 | ˃ 0.5 | ˃ 0.5 |
| H test | = 0.49 | = 0.08 | =0.07 | |
3.2. Cytological Analysis
The analysis of swabs collected from patients in both groups on the 1st day did not reveal significant differences in the cellular composition on the surface of the postoperative wound after the surgical procedure. The swabs showed the presence of neutrophils, erythrocytes, and epithelial cells with dystrophic changes characterized by cell deformation, vacuolization of the cytoplasm, and abrasion of the contour of the nuclear membrane.
By the 3rd day after surgery, the process of acute inflammation predominated in all patient groups based on swabs, with neutrophilic leukocytes being the dominant cellular composition. A trend toward a decrease in the number of granulocytes was identified in patients of Group I.
On the 5th day after surgery, the number of viable cells in the swabs of the patients of Group I was significantly higher than the number of dystrophically altered and necrotic cells. In contrast, in the patients of Group II, a large number of desquamated cells was determined, indicating fragile intercellular contacts (see Fig. 6).
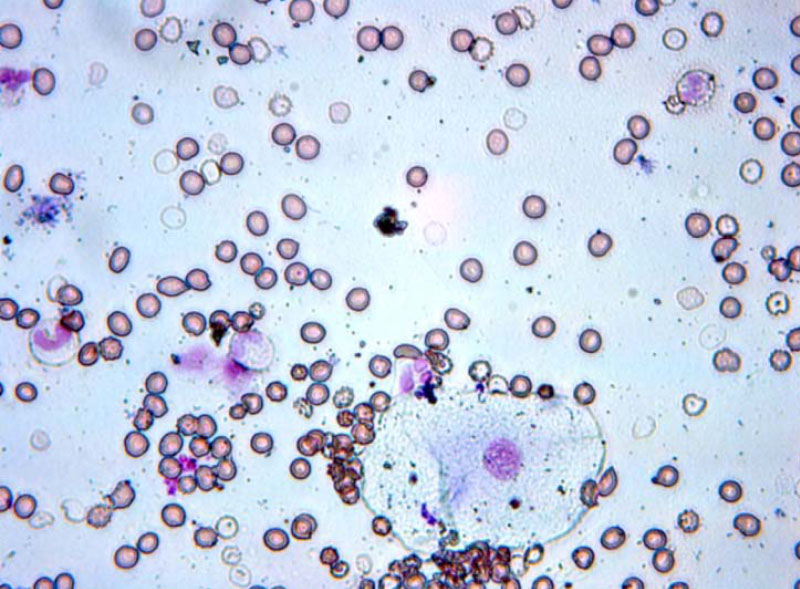
Swabs of patients of the group I on the 3rd day after surgery.
A cytological study of swabs on the 5th day after grafting showed that this period is characterized by a change in tissue reactions - from exudative to proli- ferative, since mononuclear elements dominate in the cellular composition, which is especially evident in Group I, while in Group II, most patients showed a trend towards a decrease in the proportion of neutrophilic leukocytes, which does not significantly differ from the previous study period (Fig. 7).
The swabs from patients in Group I on the 7th day after the surgical procedure showed epitheliocytes, primarily of the 5th stage of differentiation, with a constriction of the nucleus, indicating proliferative processes. Meanwhile, patients in Group II predominantly exhibited epitheliocytes in the 4th stage of differentiation (Fig. 8).
3.3. Local Immunity Analysis
The assessment of pro-inflammatory and anti-inflammatory cytokines in the peri-implant crevicular fluid revealed lower levels of TNF-α/IL-4 and IL-1β/IL-4 ratios in patients treated with PRF+SCTG grafts compared to those treated with SCTG graft only (Table 4) on days 1, 7, and 30. These changes were accompanied by observable healing, as indicated by the results of the cytology. The positive changes in cytokine levels in patients of Group I could correspond to the anti-inflammatory effect of L-PRF.
3.4. Gingival Blood Flow Analysis
The laser Doppler flowmetry results demonstrated differences between the values obtained in each group, indicating that the mean flow measured from the patients of Group I was lower when compared to the patients of Group II. Significant differences between the baseline, 1, 7, and 14 days after the surgical procedure were found in relation to gingival surgery with the PRF+SCTG and SCTG.
The obtained results after grafting indicated changes in gingival micro-vascular blood flow. Specifically, the results demonstrated a decrease in perfusion in all three groups 1 day after the surgical procedure. Additionally, the micro-vascular blood flow increased significantly 7 days after surgery, particularly in patients of Group I. It almost returned to the initial value 14 days after grafting, whereas indicators of blood flow in Group II remained at a higher level, suggesting that healing was not complete by this period of time.
The recorded fluxes in patients of Group II were significantly higher compared to the values obtained in patients of Group I (p < 0.001), indicating better modification in the vascular blood flow response after using the PRF+SCTG surgery method (Table 5).
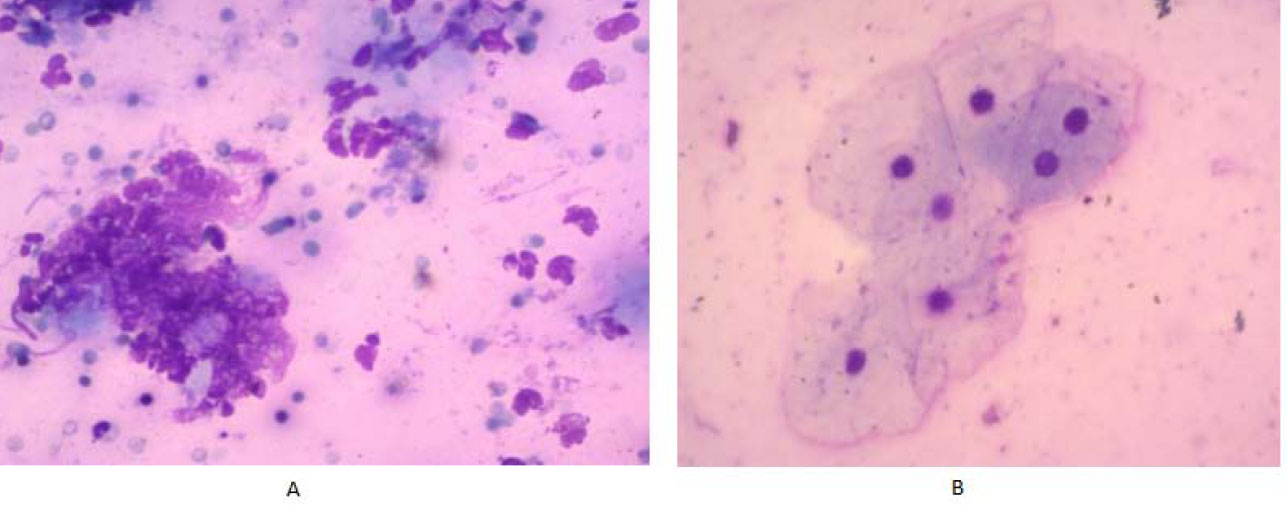
Swabs of patients of the group I (A) and the group II (B) on the 5th day after surgery.

Swabs of patients of the group I (A) and the group II (B) on the 7th day after surgery.
| TNF-α/IL-4 | ||||
|---|---|---|---|---|
| Group | 1st Day | 7th Day | 30th Day | |
| Group I PRF+SCTG [n = 47] |
4.9 | 2.2 | 0.58 | |
| Group II SCTG [n = 45] |
5.4 | 3.1 | 0.7 | |
| Group III control [n = 20] |
5.3 | 3.2 | 0.8 | |
| p value | U test | <0.05 | <0.05 | <0.05 |
| H test | >0.1 | <0.1 | <0.05 | |
| IL-1β/IL-4 | ||||
| Group I PRF+SCTG [n = 47] |
3.0 | 0.9 | 0.6 | |
| Group II SCTG [n = 45] |
4.5 | 1.3 | 0.9 | |
| Group III control [n = 20] |
4.5 | 1.2 | 0.8 | |
| p value | U test | <0.05 | <0.05 | <0.05 |
| H test | >0.1 | <0.1 | <0.1 | |
| - | Baseline | 1 Day | 7 Days | 14 Days | |
|---|---|---|---|---|---|
| Group I PRF+SCTG [n = 47] |
230 | 150 | 240 | 220 | |
| Group II SCTG [n = 45] |
220 | 140 | 290 | 250 | |
| Group III control [n = 20] |
235 | 130 | 270 | 300 | |
| p value | U test | ˃ 0.5 | ˃ 0.5 | <0,05 | < 0.001 |
| H test | ˃ 0.5 | ˃ 0.5 | <0,05 | < 0.001 | |
| - | Group I PRF+SCTG [n = 47] |
Group II SCTG [n = 45] |
Group III Control [n = 20] |
p-value |
|---|---|---|---|---|
| PES | 13.1 ± 0.02 | 11.3 ± 0.08 | 7.6 ± 0.07 | < 0.1 * < 0.1 ** |
** - H-test for all Groups.
3.5. Pink Aesthetic Score Analysis
The study results revealed a significant improvement in the Pink Esthetic Score (PES) with both grafting options 6 months after the prosthetic phase. The score measure- ments were notably better in patients treated with PRF+SCTG [13.1 ± 0.02] than in those treated with SCTG only [11.3 ± 0.08]; the difference was statistically significant with a p-value of < 0.1 (see Table 6).
4. DISCUSSION
The success of implant therapy is closely associated with healthy and stable peri-implant tissues [48]. The gingival biotype is an essential factor correlated with positive dental implant outcomes. Peri-implant soft tissue augmentation is typically recommended as a preventive strategy to lower the probability of issues and alterations to the soft tissue margin [7].
Numerous studies have underscored the significance of the thickness and width of keratinized tissue around the implant in enhancing aesthetic outcomes, maintaining soft tissue stability, and preventing peri-implant inflammation [49, 50].
For instance, Mancini et al. [51] reported better improvement in gingival thickness with the addition of a subepithelial connective tissue graft to the coronally advanced flap compared to platelet-rich fibrin. Additionally, recent studies by Sharafuddin et al. [52] and Nahil Nabil Al-Berry et al. [10] demonstrated that a connective tissue graft in combination with PRF enhances tissue biotype during immediate implantation.
These findings are consistent with our results, which demonstrated an increase in the thickness of the keratinized mucosa. Notably, the current study investigated the effectiveness of a combination of SCTG and L-PRF after the 2nd stage of delayed dental implantation in patients with a thin biotype.
In comparison with the known results of changes in mucosal thickness and PES as obtained by Sharafuddin et al. [52] during soft tissue augmentation around dental implants, our current study obtained similar data, with the difference that the surgery was performed during the second stage of implantation. In terms of the results of thickness and PES, Group I in our study had better results at 6 months after surgery (1.86±0.17) than the test group (1.75±0.69) in the study by Sharafuddin et al. Furthermore, the clinical assessment of the thickness of the keratinized tissue in the analyzed studies was conducted through transmucosal probing, whereas the present study relied on CBCT.
This current study represents the first clinical trial utilizing a parallel arm design to compare whether SCTG + L-PRF could be an effective treatment option when compared to SCTG only in soft tissue augmentation during the second stage of dental implantation in patients with a thin gingival biotype. Additionally, a control group, which received no augmentation materials, was included to isolate and understand the specific impact of SCTG and L-PRF better on the treatment outcomes. The findings suggest that SCTG + L-PRF may provide a treatment alternative that can result in a more significant impact on the improvement of soft tissue condition, as it has the potential to enhance the thickness of gingiva, improve gingiva micro-vascular blood flow, prevent complications due to the anti-inflammatory properties of L-PRF, and improve aesthetic outcomes of dental implantation.
This represents a meaningful departure from previously published literature, where studies often compared groups that used only PRF or only SCTG. Prior research has shown better outcomes in increasing the thickness of the gingival phenotype in groups of patients where SCTF was applied. It was also suggested that PRF could be used as an effective alternative to SCTG [53].
The TNF-α/IL-4 and IL-1β/IL-4 cytokine ratios in the peri-implant cervicular fluid have been assessed in numerous studies in healthy implants and peri-implantitis [54-56]. Past research has focused on pro-inflammatory cytokine levels, such as IL-1β, TNF-α, and IL-6, which can play a role in inflammation initiation and bone resorption. This study found that the ratio of pro-inflammatory and anti-inflammatory cytokines was significantly lower in Group I, which could correspond to the anti-inflammatory effect of L-PRF. Determining the ratio of cytokines could be used as an adjunct prognostic tool to clinical parameters for predicting complications and peri-implantitis.
Additionally, the PES was used to assess the aesthetic results, and it is considered a reliable instrument for assessing the soft tissue surrounding implant crowns from an aesthetic standpoint. From the results of Sharafuddin et al., the PES score in this current study significantly increased three months after the final repair placement [52].
LDF was found to be beneficial in evaluating gingival recovery post-soft tissue augmentation. LDF can provide valuable information regarding microvascular changes during the healing period [57, 58].
The variation in flux changes at 7 and 14 days may be attributed to the use of L-PRF in Group I. No previous studies were found that specifically assessed micro- vascular flow after the use of L-PRF and SCTG.
The results of the study support existing evidence on the efficacy of SCTG in peri-implant soft tissue augmentation. Previous studies, such as those by Sharafuddin et al. [52] and Al-Berry et al. [10], have indicated that combining PRF with SCTG can enhance tissue biotype and aesthetic outcomes during immediate dental implantation. However, the current study adds to this body of knowledge by showing that the combination of L-PRF and SCTG during the second stage of implantation not only enhances the thickness of keratinized tissue but also improves the overall aesthetic results and accelerates healing.
The findings of this study suggest that L-PRF combined with SCTG could be a superior option for peri-implant soft tissue augmentation, particularly in cases where aesthetic outcomes and soft tissue stability are critical. However, the study also opens up several avenues for future research. It could be interesting to test SCTG + L-PRF or conventional SCTG in combination with other preventive therapies such as Ozone [59], photobiomodulation [60], and probiotics [61] in order to understand their mutual effect on tissue healing.
Despite the promising findings, this study has several limitations. The follow-up period was restricted to six months postoperatively, which may not capture long-term outcomes such as the stability of keratinized tissue thickness and the persistence of aesthetic improvements. The study did not include patient-reported outcomes, which are crucial for understanding the full impact of the interventions from the perspective of the patient.
CONCLUSION
Based on the findings of our investigation, we conclude that the gingival phenotype thickened as a result of both SCTG and L-PRF+SCTG use. However, SCTG displayed superior performance in that regard. Despite this, given the promising outcomes observed with L-PRF+SCTG, it can be considered a viable alternative to only SCTG in peri-implant soft tissue augmentation, thereby enhancing the final aesthetic results and improving patient comfort and satisfaction by reducing the associated morbidity of the second surgical site.
To understand the causes of these variations in correlation to the pathophysiology of L-PRF and SCTG, additional research on this subject is necessary. Furthermore, in the pursuit of enhanced outcomes, experimentation with various PRF membrane layers or thicknesses is recommended.
AUTHORS’ CONTRIBUTIONS
A.A.: Data collection was done; Y.M.: The paper was written; A.E and K.T.T.: Data Analysis or interpretation was done; Z.U. and U.M.: The study concept or design was given.
LIST OF ABBREVIATIONS
| SCTG | = Sub-epithelial Connective Tissue Graft |
| KTT | = Keratinized Tissue Thickness |
| TGF-1 | = Transforming Growth Factor-beta1 |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was approved by the Research Ethics Committee of the Asfendiyarov Kazakh National Medical University (Approval number: No 6(112) from May 26, 2021). The trial was registered in www.isrctn.com (registration number ISRCTN27670172).
HUMAN AND ANIMAL RIGHTS
All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation [institutional and national] and with the Helsinki Declaration of 1975, as revised in 2013 [http://ethics.iit.edu/ecodes/node/3931].
CONSENT FOR PUBLICATION
Written consent was obtained from all parents/legal guardians of participants enrolled in the study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the ISRCTN registry at https://www.isrctn .com/ISRCTN27670172?q=ISRCTN27670172&filters=&sort=&offset=1&totalResults=1&page=1&pageSize=10, reference number ISRCTN27670172”.


