All published articles of this journal are available on ScienceDirect.
Horizontal Augmentation Using Customized Zirconia Membrane: A Case Report
Abstract
Background
Horizontal bone defects are frequently observed after tooth extraction, primarily resulting from the physiological resorption of the alveolar ridge. Reconstruction of horizontal defects is essential before or during dental implant placement, particularly in the esthetic zone. This case report aims to evaluate the effectiveness of horizontal alveolar ridge augmentation utilizing a customized zirconia membrane.
Case Presentation
A 20-year-old female patient desired to replace the missing right upper central incisor with a dental implant. The radiographic evaluation showed a concave bone defect, which makes it impossible to place a dental implant. The treatment plan was made for localized ridge augmentation using a customized zirconia membrane (CZM) prior to dental implant placement.
Conclusion
The customized Zirconia membrane is an effective and very satisfactory treatment option in the management of horizontal defects. Customized zirconia membranes reduce surgical time, facilitate the procedure for the patient and the practitioner, reduce the rate of complications, and achieve good horizontal bone gain.
1. INTRODUCTION
Guided bone regeneration (GBR) is a predictable and well-established approach to managing horizontal and vertical bone defects [1]. The biological basis of this technique depends on placing a barrier between the bone defects and surrounding soft tissues, which prevents early non-osseous cell colonization of the defect area [1]. Guided bone regeneration success requires four conditions, including (the PASS principle): primary wound closure, angiogenesis, space maintenance, and stability of bone graft materials [2].
GBR techniques involve the utilization of absorbable or non-absorbable membranes [3]. The materials utilized to fabricate these membranes exhibit a range of physical and biological properties [3, 4]. The optimal material should be biocompatible, possess adequate mechanical strength to maintain space for new bone formation, and be pliable and adjustable to conform to the shape of the bone defect [5]. Commercially available membranes come in standard dimensions and shapes, with pre-determined character- istics specified by the manufacturer. Standard titanium meshes are commonly employed as space-maintenance devices in guided bone regeneration procedures [6, 7].
Significant technological advancements in additive and subtractive manufacturing techniques have enabled the production of individualized membranes tailored to the morphology and size of the bone defect [8]. Titanium was the first material used in the manufacture of customized membranes [9, 10]. Customized titanium meshes have demonstrated their effectiveness in guided bone regene- ration procedures by reducing surgical time, enhancing clinical application convenience, and minimizing complication rates [8, 9].
Zirconia is a polycrystalline, bioinert ceramic material with excellent mechanical properties [11]. It can be easily manufactured using a standard 5-axis laboratory mill, which is readily available in most laboratories [12]. Zirconia has been widely used in the manufacture of fixed prosthetics and dental implants [13]. Therefore, zirconia is considered an ideal candidate material for the manu- facture of customized membranes. We discuss a case of localized ridge augmentation using a customized zirconia membrane (CZM) in the esthetic zone.
2. CASE PRESENTATION
The ethical approval for publication of this case report was provided by the Ethical Review Board of Tishreen University Hospital with Number 2605 on 20 Feb 2024. This study was conducted in accordance with the Helsinki Declaration, which was revised in 2013.
A 20-year-old female patient presented to the Oral and Maxillofacial Surgery department at Tishreen University Hospital with a chief complaint: A desire to replace the right upper central incisor with a dental implant. The patient stated that the loss of the right upper central incisor was due to previous trauma.
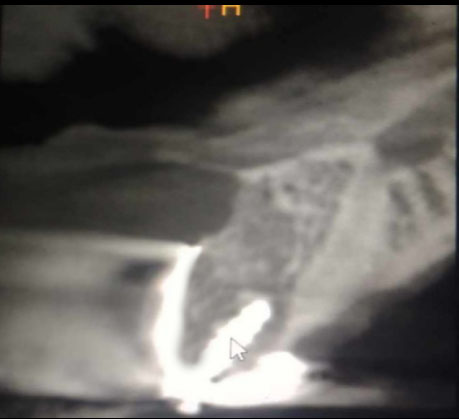
After completing the orthodontic treatment for the purpose of aligning the teeth and providing the necessary space to replace the right upper central incisor, the patient was recommended an orthopantomogram x-ray (OPG) and Cone Beam Computed Tomography scan (CBCT) for planning the implantation. OPG showed an appropriate mesiodistal distance for dental implant placement. A cone beam computed tomography (CBCT) scan showed a concave bone defect, which makes it impossible to place a dental implant (Fig. 1). The treatment plan was made for localized ridge augmentation using a customized zirconia membrane (CZM). According to Chiapasco's [8] classification of alveolar ridge bone defects, this bone defect is classified as Class 3, which requires two stages, as it is not possible to place the implant and perform the bone augmentation in one stage.
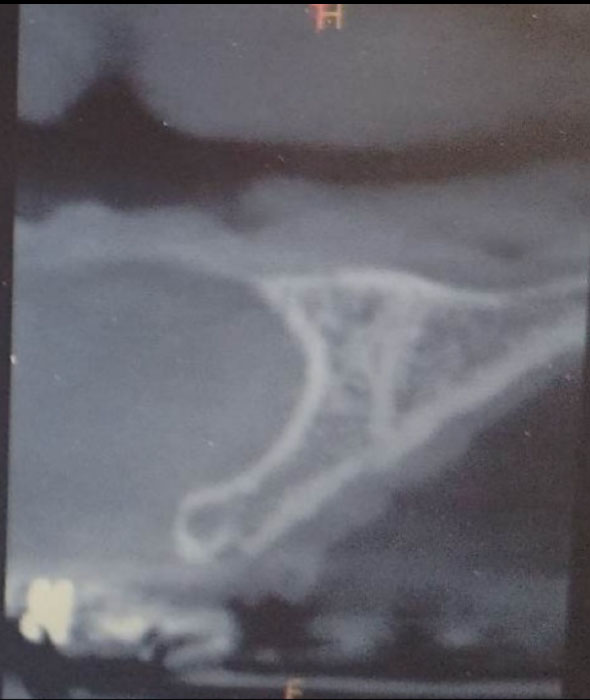
CBCT scan before surgery showed a concave bone defect.
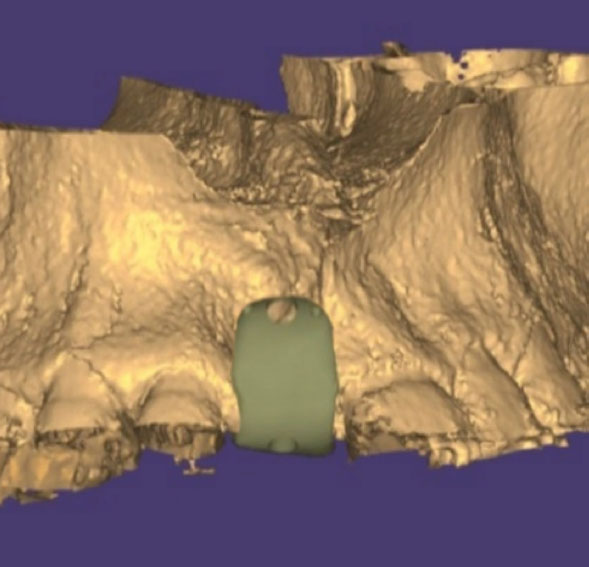
Design of CZM at a virtual model.
The customized zirconia membrane (CZM) was designed and manufactured in the Limou dental laboratory in Damascus. The design and manufacturing were performed with the aid of Exocad Software (Exocad Gmbh, Germany). The customized zirconia membrane was milled from a zirconia disc (Dental Direct®, Germany) using a 5-axis milling machine (Roland DWX 52®, Japan) (Fig. 2). The membrane was cleaned and then sterilized in an autoclave (121°C for 15 minutes).
The membrane was designed to meet the following conditions: 1) The thickness of the membrane is 0.5 to 1 mm. 2) The membrane rests on three bone margins only, mesial and lateral to the buccal bone defect area and on the lingual plate, to achieve the principle of support and correct fit for the placement of the membrane. 3) The membrane extends at least 5 mm lingually. 4) The membrane is solid without any porous structure, except for the fixation holes.
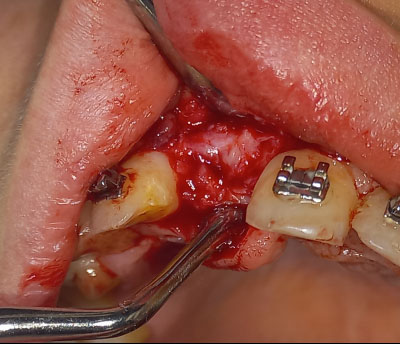
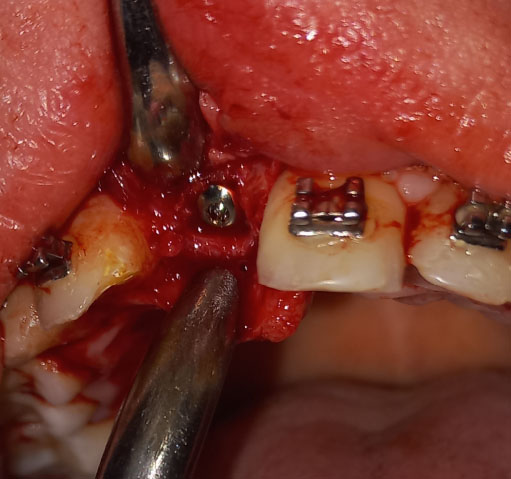
Under local anesthesia with 2% lidocaine solution and 1:100,000 epinephrine (Lidocain, AVENZOR, Syria), a full-thickness flap mucoperiosteal was reflected using one crestal incision and two vertical incisions to expose the defect area (Fig. 3). The membrane was inspected at the site of the defect to ensure it fits well with the bone margins surrounding the bony defect. A perfect fit was observed right away, with no need for any adjustments. The decortication of the cortical bone at the defect area was performed by several perforations using a 0.5 mm round bur. An allogeneic bone graft material (Cortical Cancellous powder; TRCIR Co, Iran) was moistened with sterile saline and then placed over the bone defect. The membrane was placed over the bone graft material and checked again before the fixation. The membrane was fixated using a 1.5 mm self-tapping titanium screw (Fig. 4). The vertical incisions were extended apically, the periosteum was scored, and the flap was mobilized to permit a tension-free primary closure. The flap was closed using simple interrupted suturing with 4-0 silk sutures (SilkoMed; MedSilk GmbH, Germany). Patients were prescribed amoxicillin/clavulanate 875/125 mg (Augmentin 1000, Maatouk Pharma, Syria) twice daily for 5 days, potassium diclofenac 50 mg (Flam K, Asia Co, Syria) as required, and antimicrobial mouthwash (Fresh Mouth, BIOGHAR, Syria) for 7 days. The healing was uneventful, without any complications. Sutures removal was done after 10 days (Fig. 5).
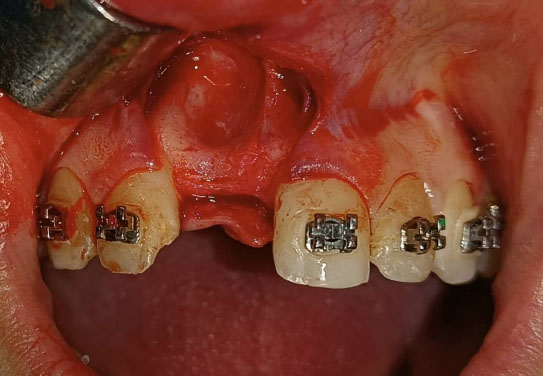
Surgical exposure of the bone defect area.
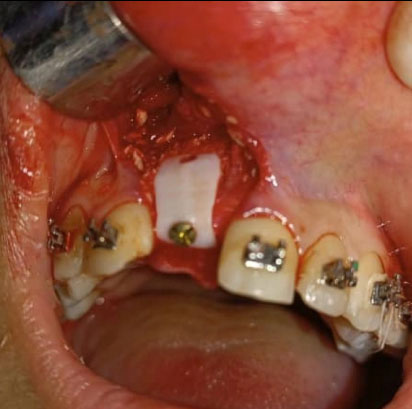
The customized zirconia membrane was fitted in position over the bone graft material. CZM was fixed to the bone with a single self-tapping titanium screw.
CBCT scans were taken 6 months postoperatively to evaluate gained bone width. The preoperative and 6-month postoperative mean width was 3.15 mm and 8.7 mm, respectively (Fig. 6A). The alveolar width gain was 5.55 mm. The customized membrane was removed after 6 months (Fig. 6B). A 3.5 mm diameter submerged dental implant (CMI IS-III Active implant; Neobiotech Co., Seoul, Korea) was inserted into the newly formed bone (Fig. 6C).
Soft Tissue healing after 4 weeks.
CBCT scan 6 months after surgery.
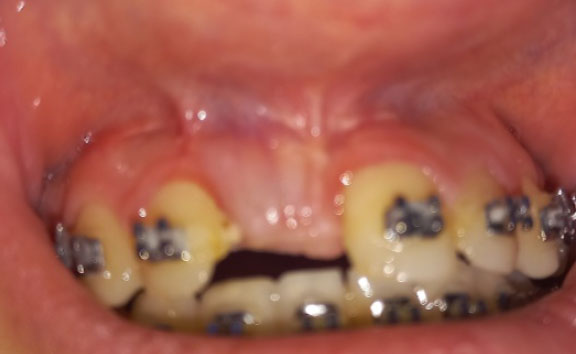
Clinical view after 6 months.
A 3.5 mm diameter submerged dental implant was inserted into the newly formed bone.
3. DISCUSSION
Replacement of teeth in the esthetic zone necessitates a thorough evaluation of both clinical and radiographic findings in order to achieve optimal results, particularly in terms of aesthetics. Tooth loss leads to inevitable resorption of alveolar bone in all dimensions, especially in the horizontal direction, leading to gingival tissue collapse and recession [14]. Traumatic extractions, advanced periodontal disease, and trauma can result in significant bone resorption, posing challenges for placing dental implants and the need for bone augmentation procedures [14]. Even when bone width is adequate for dental implant placement, bone augmentation may be required to restore the gingival contour and achieve optimal cosmetic outcomes.
The interest in bone grafting materials and techniques has grown significantly following the introduction of intraosseous dental implants. These implants necessitate a sufficient quantity and quality of existing bone, often leading to the need for bone grafting procedures, particularly in the esthetic zone. Horizontal bone augmentation techniques can be categorized into five main methods, which include bone block graft technique, guided bone regeneration, osteoperiosteal flaps, distraction osteogenesis, and expansion and splitting techniques [15].
Autogenous bone grafts are still the gold standard for managing alveolar bone defects [16]. However, Bone augmentation using autogenous bone blocks has several drawbacks, such as the need for a donor site, a limited amount of harvested bone, and the necessity to customize the bone block to match the size and morphology of the bone defect [17]. Osteoperiosteal flaps and distraction osteogenesis are intricate and delicate techniques that demand specific conditions, significant surgical expertise, and patient cooperation [18, 19]. They also carry a high complication rate and necessitate costly devices. Alveolar ridge expansion and splitting techniques are predictable and well-documented in the literature, typically yielding the desired outcomes. However, these techniques offer relatively limited bone gain, are unsuitable for severe bone defects, and necessitate specific conditions concerning bone morphology and quantity [20].
Guided bone regeneration techniques utilizing barrier membranes remain highly favored among practitioners [1, 21]. Authors have introduced new materials and techniques in recent decades to enhance bone formation, decrease complication rates, simplify surgical procedures, and lower costs [1, 21]. Titanium-reinforced PTFE membranes and standard titanium meshes have been effectively utilized in treating horizontal bone defects. They are biocompatible and possess strong mechanical properties, maintain the necessary space for new bone formation, and prevent the collapse of surrounding soft tissue into the bone defect [22].
Titanium meshes are effectively employed in conjunction with different bone-grafting materials in guided bone regeneration procedures [23]. Authors have highlighted the efficacy of this approach in managing both horizontal and vertical defects, offering superior space maintenance and resilience to soft tissue compression compared to other membrane types [23, 24]. The primary drawback of titanium mesh is its high cost compared to other membrane types. Additionally, it necessitates a relatively lengthy adaptation period to fit the morphology and size of the bone defect, along with its inherent porosity, which compromises its barrier function [25].
Exposure of titanium mesh to the oral environment represents one of the most prevalent and severe complications [26]. This issue often results in graft failure and graft material loss and typically necessitates immediate removal of the membranes to prevent infection [26]. Tension closure of the flaps, sharp edges of the titanium mesh, poor adaptation to the morphology and size of the defect, and lack of surgical experience are all factors that may contribute to membrane exposure to the oral environment [26]. To prevent this complication, various methods have been proposed, such as covering the mesh with an absorbable membrane, opting for titanium-reinforced membranes, or adopting customized titanium mesh, the latter being a more recent approach [22, 26]. Customized titanium membranes offer several advantages, such as reducing surgical time, enhancing accuracy in reconstruction, facilitating work, providing tailored fitting, featuring smoother edges, and lowering exposure rates [27, 28].
Zirconia possesses numerous biological and mechanical properties that have led researchers to recommend its utilization in bone regeneration procedures [29]. Zirconia can be viewed as an innovative material well suited for GBR due to its high biocompatibility, which results in a reduced inflammation response and lower biofilm adhesion compared to titanium [11]. Customized zirconia membrane can be easily manufactured using a standard 5-axis laboratory mill, commonly available in most laboratories. This is unlike additively manufactured customized titanium membranes, which necessitate expensive printers [25].
Despite the concave nature of the bone defect managed in our case, which is typically favorable for bone augmentation procedures and enhances the success rate of bone regeneration, the horizontal bone gain achieved was substantial, satisfactory, and exceeded that reported in previous studies [25, 29-31].
Customized Zirconia membranes can also be utilized to guide future dental implant placement. In our case, the position of the fixation screw was designed to align the anticipated position of the dental implant.
Unlike titanium, ceramic materials are brittle and cannot be bent, making it challenging to adapt them in situ when design and manufacturing inaccuracies occur. Extreme caution is necessary when tightening the fixation screw [25, 29-31]. In our study, two customized membranes were produced using identical designs to avoid the risk of breakage during fitting or fixation.
The porosity of barrier membranes remains a topic of significant controversy among authors [32-34]. Some authors have noted that fibrous tissue may infiltrate mesh apertures, causing potentially uncontrolled and unpredictable outcomes in directed bone regeneration procedures [34]. Conversely, some authors have proposed that perforations play a crucial role in facilitating the delivery of blood supply, osteoblast cells, and growth factors from the periosteum [33, 34]. In our case, we employed a solid, non-perforated customized Zirconia membrane to ensure complete isolation of the bone graft material from the oral environment in case of membrane exposure, as well as to simplify the subsequent membrane removal process.
The limitations of our case include:
1- The concave nature of the bone defect, which is typically favorable for bone regeneration.
2- Lack of no histological study of the new bone formed.
3- Absence of follow-up post dental implant placement.
CONCLUSION
Within the limits of our study, we can conclude that the customized Zirconia membrane is an effective and highly satisfactory treatment option for managing horizontal bone defects. These membranes streamline surgical procedures, enhance patient and practitioner experience, lower complication rates, and result in significant horizontal bone gain.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| GBR | = Guided Bone Regeneration |
| CZM | = Customized Zirconia Membrane |
| OPG | = Orthopantomogram X-ray |
| CBCT | = Cone Beam Computed Tomography |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The ethical approval for publication of this case report was provided by the Ethical Review Board of Tishreen University Hospital, Syria with Number 2605.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this case report and accompanying images.


