All published articles of this journal are available on ScienceDirect.
In vitro Evaluation of the Stain Removal Efficacy between Two Whitening Dentifrices during Orthodontic Treatment
Abstract
Background
This in-vitro study aimed to assess and compare the efficacy of a newly developed whitening dentifrice, Dentaklin White (TG), to Colgate Total® Whitening (CG), utilising a toothbrushing simulator machine.
Methods
Twenty enamel specimens were prepared and randomly divided into CG and TG. Orthodontic brackets were attached to the enamel specimens and were then stained with a mixture of coffee, tea, and chlorhexidine. A short 0.019x0.025” stainless steel (SS) archwire was ligated onto the brackets subsequently. All the specimens were subjected to a toothbrushing simulator for specific duration. The CIE L*a*b* colour change (ΔE) was evaluated using digital image analysis at T1 (2 weeks), T2 (4 weeks), and T3 (12 weeks) compared to T0 (baseline). Archwire roughness was analysed using a profilometer at T4 (2 years) compared to T0.
Results
The results indicated that there was a significant change in tooth colour associated with brushing duration for both CG and TG (p<0.05) with no significant difference between the two dentifrices (p>0.05). Both dentifrices notably removed more stains at Site A (p<0.05) and increased wire surface roughness (p<0.05) without a statistically difference between them (p>0.05).
1. INTRODUCTION
The global increase in aesthetic dental treatments, including tooth whitening and orthodontic procedures, is evident in Malaysia as well [1, 2]. Dental stains are categorized into extrinsic and intrinsic types. Extrinsic stains are caused by compounds incorporated into the tooth surface, such as from coffee, tea, and tobacco, or by chemical interactions with substances like cationic antiseptics and metal salts [3], particularly on rough and porous enamel. Although professional prophylaxis using paste, pumice, and air polishing can remove extrinsic stains, their development on the labial surface of front teeth can be unpleasant, especially for those undergoing orthodontic treatment.
Among active ingredients commonly found in whitening dentifrices include hydrogen peroxide or carbamide peroxide, which serve as bleaching agents to break down stains on the enamel and dentin layers of the teeth [3, 4]. Additionally, enzymes like papain and bromelain, derived from papaya and pineapple, help break down protein-based stains on the teeth. There are also stain removal agents such as polyphosphates and tetrasodium pyrophosphate, which work by preventing future staining and inhibiting the formation of tartar and stains by reducing the adherence of stain-causing compounds to the teeth. These products are marketed to address tooth staining and discoloration. Whitening dentifrices not only mechanically remove stains but also decrease the absorption of new stains. The convenience of use and cost-effectiveness compared to dental visits contribute to the growing popularity of whitening dentifrices in the market.
Progress in research technology has resulted in a rise in the creation and availability of tooth whitening dentifrices with different formulations such as those containing sodium bicarbonate [4]. Apart from its stain removal benefits, sodium bicarbonate also offers additional advantages when used in dentifrices. It can reduce the acidity of dental plaque, leading to a decrease in cavities and aiding in the remineralization of early-stage carious [5]. Moreover, the antibacterial properties of sodium bicarbonate have been extensively studied, showing positive effects on oral pathogens and promoting gingival health [6].
The stain-removing ability of a dentifrice is typically linked to its level of abrasiveness, which, at higher levels, can lead to undesirable tooth wear [7]. In this study, a newly developed whitening dentifrice with sodium bicarbonate (Dentaklin White) is compared to a marketed whitening dentifrice with hydrated silica (Colgate Total® Whitening). The clinical importance of comparing these two products lies in demonstrating that a locally produced dentifrice with lower abrasiveness can offer comparable cleaning efficacy and whitening effects to a commercially available, highly abrasive dentifrice, owing to the differences in their main ingredients. Furthermore, although the use of whitening dentifrice is common among orthodontic patients, there is also lack of data on the abrasiveness of dentifrice to the surface roughness of orthodontic arch wire. Hence, we also assessed the efficacy of a newly developed whitening and its effects on orthodontic stainless-steel arch wire.
2. MATERIAL AND METHODS
An in-vitro study was conducted (Ethical approval: JEP-2021-784) to evaluate a new locally developed whitening dentifrice, Dentaklin White, as a test group (TG) against commercially established whitening dentifrice, Colgate Total® Whitening as the control group (CG). The ingredients (taken from the sample’s package) and compositions [7] of the two dentifrices are as in the Table 1.
The study was conducted on extracted sound human premolar enamel specimens using tooth-brushing simulator machine, to assess the stains removal efficacy and orthodontic arch wire surface roughness.
PS software 3.1.2 was used to calculate the sample size. The differences in means and standard deviation (SD) of stains removal were estimated as 3.28 and 2.46 respectively as reported in a previous study Schwarzbold et al [7], utilizing 8 samples per study group. To detect the difference of 3.28 with 80% power and alpha 0.05, we needed 10 samples in each experimental group. Hence, a total sample of 20 was calculated for both test and control group (Fig. 1).
| Component | Dentaklin White | Colgate Total® Whitening |
|---|---|---|
| Active ingredient | Sodium Fluoride | Stannous Fluoride |
| Inactive ingredients | Water, Glycerin, Disodium Cocoyl Glutamate, Xantham Gum, Charcoal, Phenoxyethanol, Triethylene Glucol, Steviol Glycosides |
Water, Glycerin, Sorbitol, PEG-12, Tetrasoium Pyrophosphate, Flavour, Sodium Lauryl Sulphate, Zinc Phosphate, Cellulose Gum, Sodium Citrate, Microcrystalline Cellulose, Sodium Saccharin, Cocamidopropyl Betaine, Xanthan Gum, Citric Acid, Sucralose, Titanium Dioxide. |
| Abrasive agents | Sodium bicarbonate | Hydrated silica |
| Particle size | 5-25 μm | 15-20 μm |
| Sodium Lauryl Sulphate (foaming agent) |
Nil | Yes |
| Essential oils | Mentha Piperita (Peppermint) Oil, Orange (Citrus Aurantium Dulces) Oil, Cinnamomum Zeylanicum Leaf Oil, Eugenia Caryophyllus (Clove) Bud Oil |
Not known |
| Colour & Texture | Black, Paste | White, Paste |
| Flavour | Sweet cherry peppermint (stevia natural sweetener) |
Peppermint |
| pH (average) | 8.5 | 7.4 |
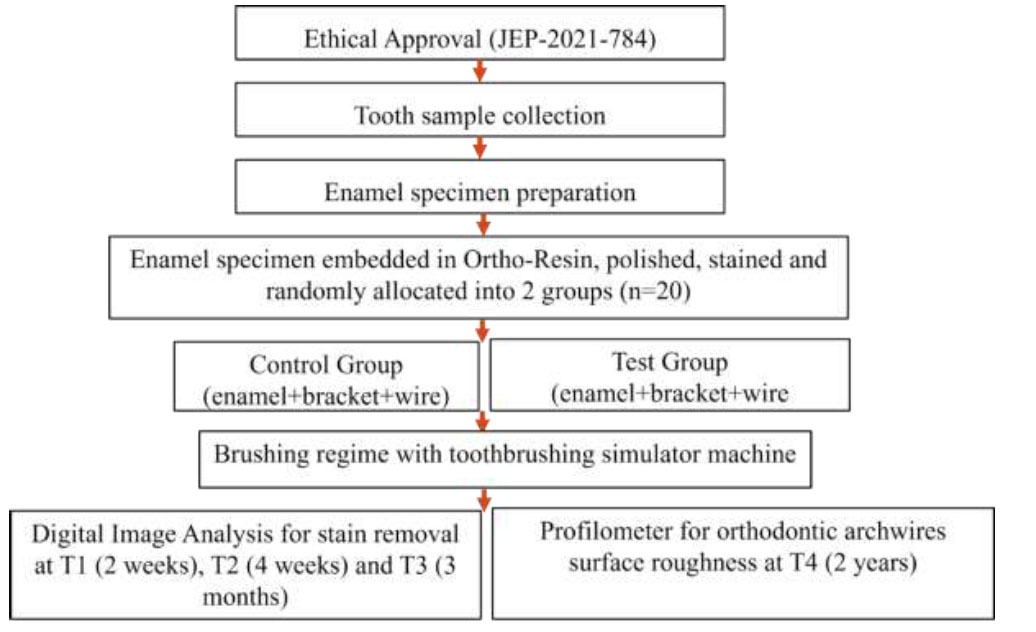
Flow chart of the experiment.
2.1. Preparation of Enamel Specimen
Extracted upper premolar teeth for orthodontic treatment purposes were collected. The crowns of the extracted upper premolars were sectioned and embedded in self-curing orthodontic resin, leaving the labial surface of enamel (enamel slab) exposed, forming a block measuring 2cmx2cmx2cm. An artificial saliva composed of 0.7mmol/L CaCl2, 0.2mol/L MgCl, 4.0mmol/L KH2PO4, 30.0mmol/L KCL, 20.0mmol 4-(2-hydroxyethyl)-1-pipe- razineethanesulfonic acid (HEPES) buffer at pH 7.0 was prepared [8]. The specimens were soaked in artificial saliva at 37 0C for 24 hours and again during the study period except for treatment and measurement periods.
Enamel slabs in CG and TG were bonded with an orthodontic metal upper premolar offset bracket (American Orthodontics, MBT prescription, Mini Master 022x028 slot, WI, USA). The enamel surface was etched with 37% phosphoric acid (3MTM ScotchbondTM Etchant, Minnesota, USA) for 30 seconds, rinsed and air dried. A uniform layer of primer (Bracepaste® MTP Primer, WI, USA) was applied to the tooth surfaces, and orthodontic adhesive (Bracepaste® Adhesive, WI, USA) was applied to the base of the bracket and positioned at the centre of the tooth surface (Fig. 2b). Excess material was removed, and light cured.
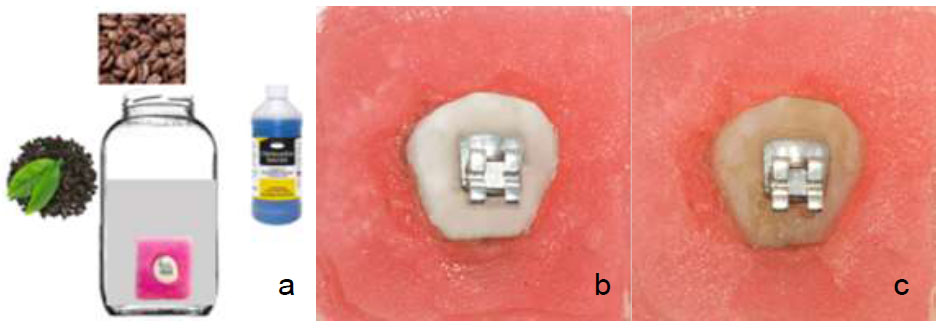
Staining broth and specimens. (a) staining broth (b) specimen before staining (c) specimen after staining.
2.2. Staining Solution and Pre-staining
Staining broth was prepared using a mixture of tea, coffee, and chlorhexidine mouthwash 0.2% as described by Schwardzbold et al [7]. The tea solution (BOH Black Tea) was prepared by brewing 1 g of tea leaves 100 ml of water for 3 min. Tea was then filtered through a fine mesh and allowed to cool down gradually to room temperature. Similar procedure was followed for instant coffee (Nescafe Classic). The tea and coffee solutions were mixed in a container. To this mixture, 30 ml of Chlorhexidine 0.12% (Oradex) was added. All specimens were immersed in this solution for 1 week, which was renewed every 24 hours and kept at 37 0C. (Fig. 2). By each broth changed, the specimens were rinsed and gently sprayed with water to remove any loose deposits.
2.3. Dentifrice Slurry
The dentifrices were used as slurries, prepared by mixing 15 g of test or control dentifrices with 45 ml of deionized water, at a ratio of 1:3 according to the EN ISO 11609:2017 standard (Dentistry-Toothpastes: Require- ments, test methods and marking) [9].
2.4. Toothbrushing Simulation
A custom-made toothbrushing apparatus, UKM (Universiti Kebangsaan Malaysia) Wear and Toothbrush Simulator (Fig. 3a, b) was used for toothbrushing simulation throughout the study. The commercially available toothbrush (Colgate® SlimSoft Orthodontic Toothbrush) was attached to the toothbrushing machine. Specimens were mounted onto the toothbrushing simulator machine with a brushing force of 150g in an axial movement.
2.5. Brushing Regime
The average time taken to brush twice daily by a person was 240 seconds. Based on this estimation, the maximum contact time for one tooth surface per day was 10 seconds. Thus, the total brushing time is calculated to be 60 minutes, which is equivalent to 1 year of brushing [10]. The toothbrush was replaced after 45 days [9] and the dentifrice slurry was changed every 2 minutes of brushing. Data collection and analysis were done at T1=140 seconds (equivalent to 2 weeks of brushing), T2=280 seconds (4 weeks) [11] and T3=14 minutes (3 months) for stains removal assessment, and at T4=120 minutes (2 years) for orthodontic archwire roughness assessment.
2.6. Colour Analysis and Setup
The stains on enamel specimens were measured by using photography technique to obtain the CIELAB value. The specimens were placed in the photo light box and a DSLR camera (Canon EOS 600D, Macro Lens Tamron 90 mm, and YongNuo Macro Ring Lite YN14EX) was used to capture the image of specimens at a fixed distance with the same setting and surrounding (Fig. 4a). Measurements were taken on the enamel surface at six sites (Fig. 4): incisal to the bracket slot (Site 1 and Site 2), gingival to the bracket slot (Site 4 and Site 5), and mesial and distal of the bracket slot (Site 3 and Site 6) before and after treatment (Fig. 4b). The CIE L*a*b* colour scale was gained by using Adobe Photoshop (Version 21.2.4.323) whereby the images at different time points (T0, T1, T2, and T3) for the same sample were overlapped and six different fixed points were selected for reading. Three readings were repeated at each site and overall mean value was obtained. The difference between the pre-test and post-test readings represents the ability of the test products to remove stains. Besides that, the readings on sites 1, 2, 4, and 5 were averaged (Site A) and compared to the mean value of sites 3 and 6 (Site B) to compare the stain removal efficacy at different sites of enamel specimens. The overall change in stains was calculated using the following CIE L*A*B* equation, ΔE= {<LT1/T2/T3 – LT0>2 + <aT1/T2/T3 – aT0>2 + <bT1/T2/T3 – bT0>2}½
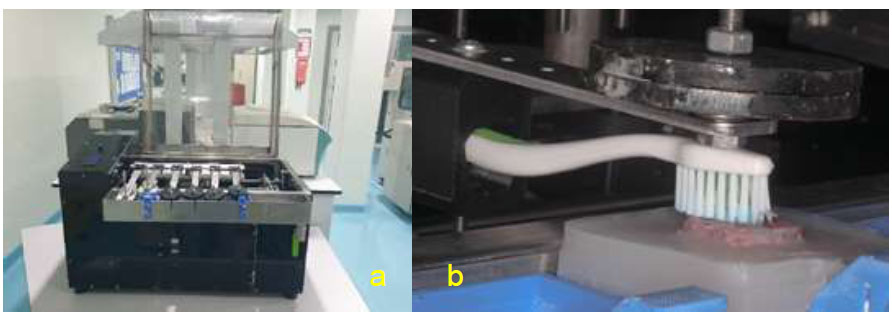
(a) UKM Wear and Toothbrush Simulator (b) close up view during simulated toothbrushing.
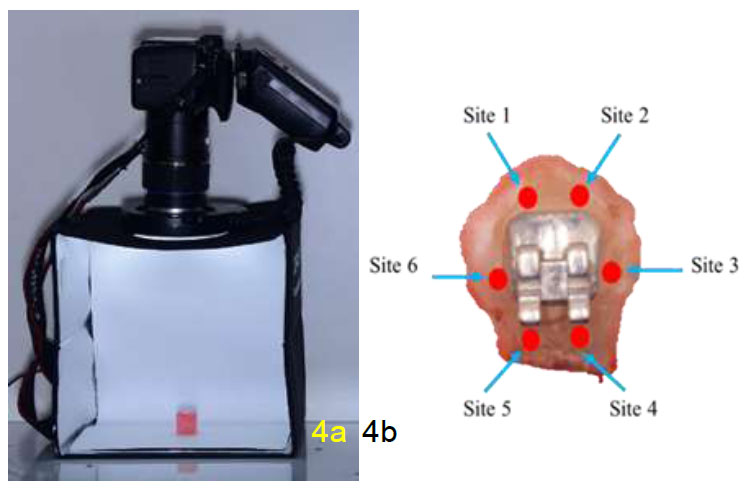
(a) Camera setting for enamel specimen picture (b) sites of enamel surfaces.
2.7. Orthodontic Wire Surface Roughness
In this study, a 0.019x0.025” SS arch wire (American Orthodontics, WI, USA) measuring 20mm was ligated to the brackets with elastomeric modules during tooth- brushing to simulate clinical scenario (Fig. 5a). This arch wire is made from the highest quality vacuum-remelted 304V stainless steel alloy. This ensures a pure, inclusion-free arch wire, with superior tensile strength and less brittleness. It has a minimum of 18% chromium and 8% nickel, combined with a maximum of 0.08% carbon. All dimensions were closely monitored with state-of-the-art laser micro meters to ensure consistent and accurate tolerances. Strict corner-radius controls ensure optimal wire-to-bracket interaction. All arches were carefully stress relieved and polished to reduce breakage and excess friction.
The wire roughness was assessed using surface profilometer (Infinite Focus Real 3D Alicona). Three fixed points were selected on each side of the wire at the 0.025” side (Fig. 5b), whereby this instrument focused on the surface at the prescribed distance and quantified roughness as Ra value. A total of three readings were gained at each specific point and were averaged to gain the mean value for one wire. The measurement was done before the enamel specimens were subjected to toothbrushing machine (T0) and after the specimens were subjected to toothbrushing regime (T4).
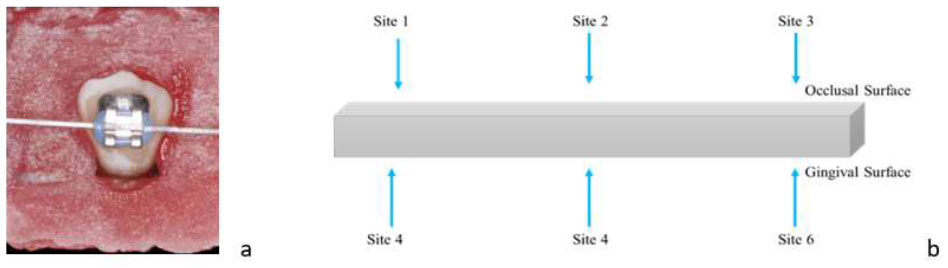
(a) 0.019x0.025” SS arch wire ligated to the brackets with elastomeric modules and (b) Six sites on orthodontic arch wire for roughness assessment.
2.8. Scanning Electron Microscopy (SEM)
The 0.019x0.025” SS archwire were sent for SEM image analysis (Zeiss, Supra 55vp) and elemental analysis to compare the elements on the wire before and after toothbrushing.
2.9. Statistical Analysis
The data analysis was done using SPSS version 26. Descriptive data was expressed as mean ± standard deviation (SD) unless otherwise stated. Paired t-test was used to compare the within group result while an independent t-test was used to compare the between-group results. A two-way ANOVA test was conducted to compare the stain removal efficacy at different time points within and between groups. A value of p < 0.05 was considered statistically significant. The normality test was performed using Shapiro-Wilk test and all the mean values fulfilled the criteria for parametric test with p-value > 0.05. The sphericity assumption was violated (p < 0.05), so the Greenhouse-Geisser correction was applied for ANOVA test.
3. RESULTS
3.1. Stain Removal Efficacy
Fig. (6) shows the colour changes of the specimen before staining, after staining and after toothbrushing. In general, the colour darkens due to staining became whiter after toothbrushing in both groups. The colour changes (ΔE) are presented in Table 2.
Two-way mixed ANOVA indicated that there was a significant effect of the time of toothbrushing on the colour changes (ΔE), p<0.001. However, types of dentifrice don't significantly affect the colour changes (ΔE) p>0.05. In addition, there was no significant interaction between types of dentifrice and time of toothbrushing regarding ΔE (p>0.05) (Table 3).
The mean and SD for ΔE according to site are presented in Table 4.
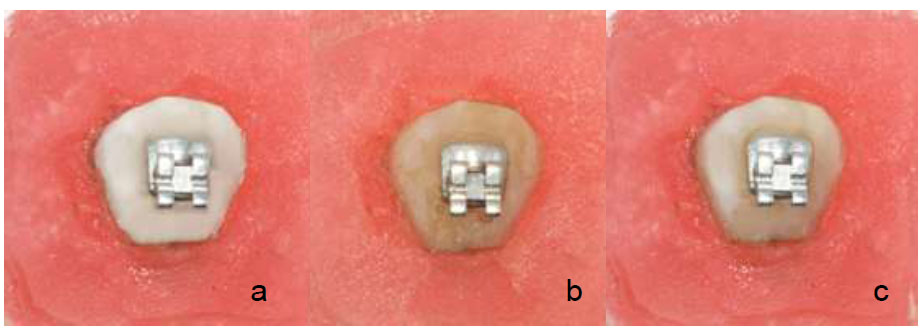
Colour changes (a) specimen before staining (b) specimen after staining (c) specimen after toothbrushing.
| Time Point | Control Mean (SD) |
Test Mean (SD) |
p-value |
|---|---|---|---|
| T1-T0 | 9.417 (2.385) | 10.185 (0.943) | >0.05 |
| T2-T0 | 12.909 (3.461) | 12.481 (2.226) | >0.05 |
| T3-T0 | 15.995 (3.947) | 15.158 (2.608) | >0.05 |
| Factor | p-value |
|---|---|
| Group (control vs test) |
>0.05 |
| Time of toothbrushing | 0.000* |
| Time of toothbrushing x Types of dentifrice | >0.05 |
| Site of Enamel | Time of Toothbrushing | Control Group | Test Group |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Site A | T1-T0 | 11.050 (3.720) | 11.086 (1.609) |
| T2-T0 | 14.756 (4.875) | 13.505 (3.274) | |
| T3-T0 | 17.422 (4.978) | 14.981 (3.331) | |
| Site B | T1-T0 | 6.148 (1.578) | 8.641 (1.294) |
| T2-T0 | 9.216 (2.133) | 10.433 (1.652) | |
| T3-T0 | 11.665 (2.754) | 13.821 (2.081) |
| Factor | Control Group | Test Group | ||
|---|---|---|---|---|
| F | p-value | F | p-value | |
| Site | 14.993 | 0.004* | 5.641 | 0.042* |
| Time of Toothbrushing | 72.253 | 0.000* | 57.830 | 0.000* |
| Site * Time of Toothbrushing | 1.079 | 0.361 | 5.450 | 0.014* |
| Time of Toothbrushing | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | p-value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| T1-T0 | 2.457 | 1.554 | -0.903 | 5.816 | 0.138 |
| T2-T0 | 2.468 | 1.954 | -1.637 | 6.573 | 0.223 |
| T3-T0 | 4.597 | 1.718 | 0.987 | 8.206 | 0.015* |
| Types of Dentifrice | CG Control Group | TG Test Group | ||
|---|---|---|---|---|
| Mean difference | p-value | Mean difference | p-value | |
| Pre-test - Post-test | -0.021 (0.012) | 0.004* | -0.018 (0.011) | 0.001* |
| Difference between CG and TG |
p = 0.499 | |||
Two-way repeated measure ANOVA indicates that there was a site of enamel (p<0.05) and time of tooth brushing (p<0.001) significantly affect the colour changes ΔE in both CG and TG (Table 5).
However, significant interaction between the site of enamel with time of brushing on the ΔE was only observed in the TG (p<0.05). The differences in ΔE between Site A and Site B were calculated. Significant main effect of the types of dentifrice on the differences in colour changes between Site A and Site B observed (p=0.003). Hence, independent t-test was carried out to determine if there was a statistical difference between the control and test dentifrice at each timepoint (T1-T0, T2-T0, and T3-T0). There was a significant difference in the colour change between the CG and TG at T3-T0, whereby the TG recorded a less mean difference of 4.597 between Site A and Site B than the CG, p=0.015. (Table 6).
3.2. Orthodontic Archwire Surface Roughness
At T4 (2 years of tooth brushing), the orthodontic wire surface became significantly rougher in CG at -0.021µm (p<0.001) and in TG at -0.018µm (p=0.001). Although the CG recorded a higher roughness value compared to TG, this difference was not statistically significant (p>0.05) (Table 7).
3.3. SEM Image of Orthodontic Archwire
The surface of a 0.019x0.025” orthodontic SS arch wires were analysed using SEM. The wire exhibited lines and grooves running parallel to the long axis of the wire, with some minor pitting visible at 1000x magnification prior to toothbrushing. After simulating 2 years of toothbrushing, both the CG and TG showed a rougher surface and texture, with visible scratches, grooves, and signs of wear along the wire. The wire surface became more irregular, but there was no residual dentifrice or debris observed on its surface (Fig. 7).
3.4. Elemental Analysis of Orthodontic Archwires
The elemental analysis of arch wire showed that there is no difference in the arch wire elemental pre-brushing and post-brushing, indicating that no obvious deposition of dentifrice elements on the arch wire that could affect the surface roughness (Fig. 8).
4. DISCUSSION
In our study, we used photography image analysis to assess the amount of stain removal in the enamel specimens. To reduce the error, the enamel specimens were allowed to dry at room temperature for 30 minutes. The images were taken using the same setting and in a dark room to prevent any additional light source that could affect the result. This technique was proven to be effective when used in both lab study [12] and clinical study [13]. The previous study used the magnetic lasso tool in Adobe Photoshop for point selection, which may result in inaccuracy of results due to different points selection on different images. To ensure high accuracy and consistency of point selection in this study, the image taken at different time points were overlapped in Adobe Photoshop at the same angle and location with six points marked on the image with the L*a*b* value. Other objective measurements of stains removal include the use of spectrophotometer and colorimeter [14]. In our study, the enamel specimens were not polished to produce a flat surface and hence the use of a spectrophotometer can be subject to errors due to the natural curvature of the enamel specimens [15].
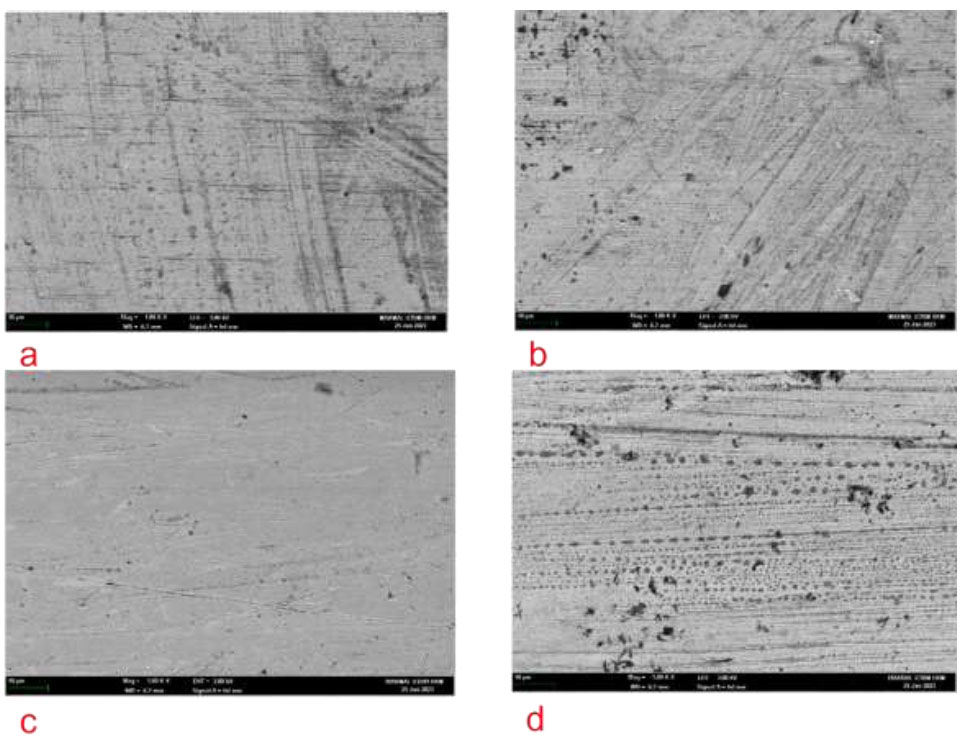
SEM image at x1000 magnification of 0.019x0.025” SS arch wire. (a) Pre-test CG (b) Post-test CG (c) Pre-test TG and (d) Post-test TG.
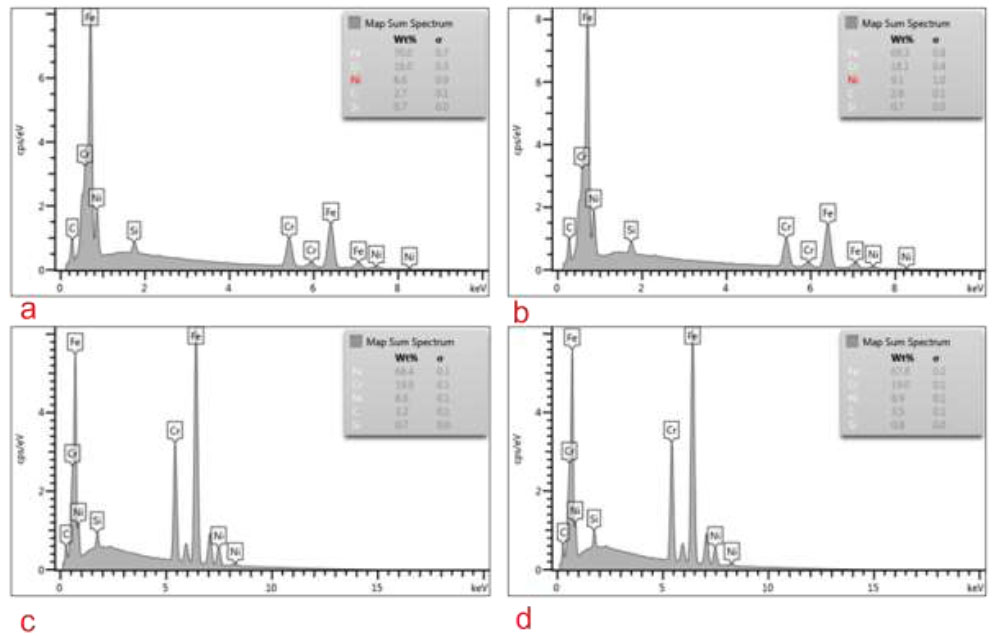
Elemental analysis of 0.019x0.025” SS arch wire. (a) Pre-test CG, (b) Post-test CG, (c) Pre-test TG, and (d) Post-test TG.
Our study found that there was no statistical difference between the TG and CG, which was in agreement with study done by Alshara et al [16]. However, study done by Schemehorn et al was in favour of hydrated silica [17]. While more studies showed that sodium bicarbonate was superior to silica in stain removal [18, 19], our study found no difference between both groups. The differences in the abrasivity of whitening dentifrices dependent on particle size, shape, hardness and possibly the pH [20]. In our study the particle sizes and pH of sodium bicarbonate in TG are similar to hydrated silica found in Colgate Total Whitening dentifrice, hence there was no difference in efficacy of stain removal between the two. In addition, the TG did not have peroxide added to further enhance the whitening effects [20]. Other than that, the insignificant result can be due to a generally lower concentration of sodium bicarbonate used in the dentifrice [21] and does not contain sodium lauryl sulphate (SLS) that is responsible for foam production and lowers the stains molecules' surface tension [22].
The stain removal efficacy of CG and TG was further subdivided into Site A and Site B, which is a modification of Ortho-Plaque Index (OPI) [23]. This is due to Site A is easily accessible to the toothbrush and whitening dentifrice in comparison to Site B. This was reflected in our results showing that Site A had significantly more ΔE than Site B in both CG and TG. This could be due to the presence of orthodontic archwire and the horizontal movement of the toothbrush resulting in less diffusion and contact of abrasive agents to the enamel surface underneath the archwire as abrasive materials is only effective in areas that a toothbrush's bristles can access [20]. The lesser differences in ΔE between Site A and Site B indicate that the ΔE between both sites is almost similar, hence a higher ΔE at Site B, which is impeded by the orthodontic wire. The significant result at T3 may be due to sodium bicarbonate having a better penetration compared to the hydrated silica. The result suggested that the use of test dentifrice is better for patients with conventional fixed appliances after the use of 3 months.
The roughness reading was gained from the depth of wire, as this is the site that is in contact with the orthodontic bracket’s slot during sliding mechanics. Hence, any changes to the orthodontic wire’s surface characteristic at this site may result in an alteration of frictional force. Both CG and TG resulted in a significant increase of archwire surface roughness at T4. Elevated surface roughness can lead to a significant rise in frictional forces due to increased contact between the bracket and wire [24]. This can result in a reduction of orthodontic force by 50% or greater, ultimately diminishing the effectiveness of orthodontic treatment and may result in anchorage loss [25]. Nevertheless, the clinical significance of the resulting increased friction force, remains uncertain.
5. LIMITATION
This is an in-vitro study involving small sample size that may not be a true resemblance to real clinical situations, for example in crowded teeth during alignment stage, types of toothbrushes and toothbrushing technique. Hence, it is suggested that future clinical trials can be carried out involving larger samples and more types of whitening dentifrices. It would be valuable to evaluate the prevention of stain uptake in the future study as well.
CONCLUSION
From our study, it can be concluded that:
1) Both Dentaklin White and Colgate Total® Whitening dentifrices had the same stain removal efficacy during orthodontic treatment.
2) The stain removal efficacy was higher at Site A compared to Site B for both Dentaklin White and Colgate Total® Whitening dentifrices with a significant difference at T3.
3) The orthodontic SS archwire became rougher after brushing with both Dentaklin White and Colgate Total® Whitening dentifrices.
Dentaklin White dentifrice had equal efficacy with Colgate Total® Whitening for stains removal during orthodontic fixed appliance treatment and slightly superior in accessing hidden areas after 3 months of use.
AUTHORS CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ANOVA | = Analysis Of Variance |
| CG | = Control Group |
| CIE L*a*b* | = Colour space defined by the International Commission on Illumination |
| DSLR | = Digital Single-Lens Reflex |
| SEM | = Scanning Electron Microscopy |
| SD | = Standard Deviation |
| SPSS | = Statistical Package for the Social Sciences |
| TG | = Test Group |
| UKM | = University Kebangsaan Malaysia |
| ΔE | = Delta E |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Research Ethical Committee of Universiti Kebangsaan Malaysia, reference number: (UKM/PPI/111/8/JEP-2021-784).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIAL
The data and materials used in this manuscript are not publicly available to ensure confidentiality and compliance with ethical standards. However, they will be made available by the corresponding author upon reasonable request, provided the requester meets the criteria for access to confidential data.


