All published articles of this journal are available on ScienceDirect.
A Comparative Analysis of Open Flap Debridement with and without Autogenous Periosteal Graft as a Barrier Membrane for the Treatment of Class II Furcation Involvement in Mandibular Molars: A Case-control Study
Abstract
Background
The current study aimed to compare the efficiency of autogenous periosteal graft (APG) as a barrier to open flap debridement (OFD) in Class II mandibular furcation defects.
Methods
A total of 24 patients participated in this research and were divided into the test group and the control group. The test group received treatment with OFD plus APG as a barrier, while the control group received only OFD. Plaque index (PI), papillary bleeding index (PBI), vertical probing pocket depth (V-PPD), vertical relative attachment level (V-RAL), relative gingival marginal level (RGML), and horizontal probing depth (HPD) were assessed at baseline, 3 and 6 months, respectively, in both groups. The normality test was performed with the Shapiro-Wilk test. Descriptive statistics were performed for V-PPD, HPD, RGM, L, and V-RAL. A comparison between baseline and 3 months and 6 months was made using the paired t-test.
Results
The PI and PBI scores were compared at baseline, 3, and 6 months in both groups, which indicated no significant differences. A comparison of clinical parameters, V-PPD, V-RAL, RGML, and HPD, from baseline to 3 and 6 months in both groups showed significant differences except for RGML from baseline to 6 months in the control group. Moreover, the comparative analysis between the control and test groups showed significant improvement in all clinical parameters.
Conclusion
APG, along with OFD as a barrier in the management of Class II mandibular furcation defects, demonstrated a significant advancement in clinical parameters.
1. INTRODUCTION
Destruction of the hard and soft connective tissue around the teeth is predominantly caused by periodontal disease. The regeneration of these damaged connective tissues could be attained with periodontal therapy.
However, the outcome of single-rooted teeth is more foreseeable than that of multi-rooted teeth [1, 2]. Periodontal condition is also influenced by a variety of prosthetic factors [3]. Furcation defect is the most challenging treatment among all periodontal treatments. Although the techniques of periodontal treatment have improved nowadays, there are some restrictions in furcation therapy due to the approachability of the furcation area. Both clinicians and patients find it difficult to remove plaque from the furcation area due to the complicated anatomical structures [4, 5]. An inadequate success rate has been observed in both surgical and non-surgical furcation defects. Therefore, it seems that only regenerative treatment could improve the furcation defect.
Different regenerative surgical approaches could be employed in the treatment of Class II furcation defects in mandibular molars. Improvement in the clinical attach- ment level and pocket depth could be attained with allografts, autografts, and Guided Tissue Regeneration (GTR) [6, 7]. However, very few studies exhibited signs of bone regeneration using porous hydroxyapatite implants [8]. Using the decalcified freeze-dried bone allograft, along with citric acid treatment combining coronally positioned flap technique, a 50% improvement in furcation defects can be achieved [7]. A limited number of successful outcomes have been reported in Class II furcation defect treatment using freeze-dried bone allografts and autografts, except for a few reports [9, 10]. Moreover, bone replacement grafts showed many complications, such as microbial contamination, epithelial exclusion, and containment of the graft.
Current techniques of GTR are based on the retardation of epithelial growth and acceleration of coronal proliferation of the periodontal ligament [11-13]. Different clinical studies tested the gingival tissue migration during the healing of the periodontal flap in periodontal defects, including furcation [11, 12]. The ability of the GTR procedure to predict new bone formation has not been established. Many studies on GTR furcation therapy have been performed extensively with non-resorbable and bioresorbable barriers. Although few studies demonstrated a significant result in improving clinical attachment, some studies reported the opposite [11, 12]. However, the GTR procedure exhibited the limitation of successful treatment outcomes irrespective of the type of membrane barrier.
The most favorable treatment for Class II furcation has been the combination of a GTR barrier and bone replacement graft [13]. Bone graft with a membrane might stabilize the blood clot and decrease the dead space under the membrane [14]. However, some clinical trials showed no significant difference between using barrier membranes and bone grafts together and using membranes only [15, 16]. Hence, improving the GTR therapy by using a replacement graft is not distinctly justified.
Stabilizing the wounds and supporting bone regeneration during the healing of Class II furcation defects, connective tissue grafts (CTGs) have been used as a protective barrier [16, 17]. Studies reported that subepithelial CTGs increase the healing at the root area, which intensifies regeneration, new bone formation, and connective tissue attachment towards the original gingival margin [18, 19]. Moreover, one previous study reported that the treatment effects of CTGs outweigh the GTR therapy in Class II furcation defects [20]. In the procedure of GTR, the application of periosteum as a barrier has not been discussed enough in the literature. Periosteal barriers improve the bone fill, which illustrates that bone formation in the furcation area could be improved with the use of a periosteal layer [15].
Most of the clinical trials on furcation defects used open flap debridement as a control group in contrast to regenerative techniques [6, 15, 21-28]. While the outcomes of the regenerative procedures were generally inferior, significant positive effects were noted. Neverthe- less, the clinical efficacy of open flap debridement (OFD) may extensively vary. This outcome is probably related to the design of the flap, incision, and sutures. Recently, an autogenous periosteal graft (APG) barrier has been used in wound protection and stabilization of the furcation site during the healing process in order to sustain the regeneration of bone in mandibular Class II furcation defects [15, 17, 20, 26, 29].
Hence, this study aimed to compare the efficacy of autogenous periosteal graft (APG) as a barrier to open flap debridement (OFD) in the treatment of human mandibular Class II furcation defects.
2. MATERIALS AND METHODS
2.1. Study Design and Population
A total of 24 patients were selected in this case control who were diagnosed with moderate to severe periodontitis and sought treatment at the Department of Periodontics, Modern Dental College and Research Centre, Indore, India.
Two different therapeutic modalities for the treatment of mandibular Class II furcation defects were compared. The patients were randomly divided into two groups. Two groups (the test group and the control group) were treated with a regenerative approach. The control group was treated with OFD only, which comprised 12 sites, and the test group was treated with OFD and APG as a barrier (OFD+APG) and comprised 12 sites.
Inclusion criteria for the current study were: Class II furcation defects present on the buccal surface of at least one mandibular molar, the tooth surface should be intact adjacent to the furcation area, horizontal probing depth of ≥ 3mm without through and through furcation, electric pulp testing showed a normal response, free of any systemic diseases, and not under medication. On the other hand, non-compliant patients, mobility of the affected tooth, previous periodontal surgical treatment at the same quadrant, and history of smoking or any other tobacco products were excluded from this study.
Patients who were selected for this study were explained all the risks and benefits of the treatment. Informed consent was acquired from all participants. This study was approved by the ethical committee of Modern Dental College and Research Centre, Indore, with the approval number IEC/MDCRC/2011-12/0037. Full mouth scaling, root planing, and oral hygiene instruction (OHI) were provided to all patients as phase I therapy. If necessary, occlusal adjustment was performed. Instruction for plaque control was delivered repeatedly until 80-85% plaque control was achieved by the patients. Six weeks after the phase I therapy, a re-evaluation examination was conducted to evaluate the patient’s cooperation and determine the need for periodontal surgery.
2.2. Clinical Assessment
Full mouth plaque score and gingival inflammation were assessed using the Turesky-Gilmore Glickman modification of Quigley-Hein 1970 plaque index (PI) [30] and papillary bleeding index (PBI) [31], respectively, before administration of local anesthesia on the first day of the surgical procedure. PI and PBI were reevaluated in the first and sixth months after the surgical procedure.
A discoloring agent was used to assess the plaque on the lingual and buccal/labial surfaces of the teeth. The criteria of PI are presented in Table 1. The total scores around each tooth were divided by two to achieve the PI score for each tooth, and the accumulation of the PI score for all teeth divided by the total teeth examined was performed to attain the PI score per person.
For measuring the papillary bleeding index (PBI), a periodontal probe (William’s calibrated Probe) was used, which was inserted into the sulcus of the gingiva at the papillary base to the mesial side. Then, the probe was moved from the coronal area to the papillary tip, and the procedure was repeated for the distal aspect. PBI was recorded as per the criteria mentioned in Table 1. PBI scores were attained per person by adding the PBI score for all teeth, which was divided by the number of total teeth examined.
2.3. Probing Measurements
Vertical probing pocket depth (V-PPD), horizontal probing depth (HPD), relative gingival marginal level (RGML), and vertical relative attachment level (V-RAL) were measured for all patients.
The baseline presurgical measurements were recorded for groups. Fabrication of an occlusal stent was performed with cold-cure acrylic resin. The stone model was obtained from the alginate impression to reduce the measurement variations for free-hand probing positioning. The tooth selected for surgery, along with the adjacent teeth, was covered by the occlusal stent. The probe was inserted with proper angulation into the pocket while the acrylic stent was in position. A pencil was used to mark the acrylic stent at the contact area between the buccal area and the probe. A low-speed bar in a cylindrical shape was used to make three grooves on the marked areas, which were used for the probe insertion guide for future visits.
V-RAL and RGML were measured from the apical border of the acrylic stent to the pocket base and to the gingival margin, respectively. V-PPD was obtained by subtracting RGML from V-RAL. HPD was recorded using a color-coded curved probe (Naber’s Probe) with markings 0-3, 3-6, and 6-9 mm. All measurements were performed at three sites (distal line angle, mesial line angle, and mid-buccal) of each furcation area. The measurements were repeated during the first, third, and sixth months after the surgery.
2.4. Surgical Procedure
Patients were asked to use the chlorhexidine mouthwash (Hexidine, ICPA Health Products Ltd, India) for 1 minute. Infection control and complete asepsis were emphasized during the surgical procedure.
2.4.1. Flap Design (incisions)
Crevicular incisions were made using Bard-Parker number 15 surgical blades after administrating the local anesthesia (2% lignocaine, epinephrine, 1:100,000). In order to attain the primary wound closure, the incision was carried out inter-proximally to reserve the interdental papillae. Two adjacent teeth of the experimental site were included in the flap.
| PI | PBI | ||
|---|---|---|---|
| Score | Criteria | Score | Criteria |
| 0 | No plaque | 0 | No bleeding |
| 1 | Separate flecks of plaque at the cervical margin of the tooth | 1 | A single discreet bleeding point appears |
| 2 | A thin, continuous band of plaque (up to 1 mm) at the cervical margin | 2 | Several isolated bleeding points/a single fine line of blood appears |
| 3 | A band of plaque wider than 1 mm but covering less than one-third of the crown | 3 | The interdental triangle fills with blood shortly after probing |
| 4 | Plaque covering at least one-third but less than two-thirds of the crown | 4 | Profuse bleeding occurs after probing; blood flows immediately into the marginal sulcus |
| 5 | Plaque covering two-thirds or more of the crown | - | |
2.4.2. Reflection of Flap
The alveolar bone was exposed with a periosteal elevator (24 G Hu-Friedy) to elevate the mucoperiosteal flap both lingually and facially.
2.4.3. Debridement and Root Surface Management
The flap surface was curetted; granulation tissue was detached from the area of the root surface, which was exposed, and scaling was done using the scaler. Root planing was performed until a smooth, hard consistency was achieved. Intra-surgical measurements were documented after irrigating the area with a physiologic saline solution and achieved hemostasis.
2.5. Surgical Procedure for the Control Group
After thorough debridement and root surface management, the envelop flap was repositioned and sutured with 4-0 Mersilk to its original position. The procedure was carefully conducted so that the furcation was completely covered (Fig. 1).
2.6. Surgical Procedure for the Test Group
2.6.1. Harvesting of the APG
The “trap door approach” was followed in order to harvest the APG palatal tissue between the maxillary canine and maxillary first molar. An incision was created apically three mm apical to the gingival margin and parallel to the maxillary premolar and 1st molar. In order to cover the entire furcation area, another perpendicular incision was made. Underlying connective tissue was exposed, and the periosteal elevator was used to remove the connective tissue. It was modified as per the required dimension after the removal of excess fatty and glandular tissue. The connective tissue graft was cleaned with saline, and the primary flap was replaced and sutured with a 3-0/4-0 Mersilk suture.
2.6.2. Securing the APG on the Recipient Site
The harvested graft was trimmed accordingly, covering 3 mm of the alveolar bone, which extends from the apical to the cementoenamel junction. The graft was then secured to cover the furcation defect such that the periosteal surface of the graft faced the furcation by resorbable 4-0 Vicryl suture using a sling suture technique. Lastly, the primary flap was replaced with a 3-0/4-0 Mersilk suture to obtain closure by using an interrupted suturing technique (Fig. 2).
2.7. Post-operative Care
The periodontal dressing was placed immediately after the surgical procedure to cover the wound. In the test group, the donor area was covered by a self-curing acrylic palatal stent. Systemic antibiotics (Amoxicillin, 500 mg, T.I.D, 5 days) and non-steroidal anti-inflammatory drugs (IBIGESIC- Ibuprofen + Paracetamol, T.I.D for 5 days) were prescribed for the patients. Patients were asked to avoid brushing at the surgical site and instructed to rinse daily with chlorhexidine mouthwash for 4-6 weeks. Patients were also asked to be careful at the surgical site to avoid any trauma. The surgical dressing was removed after a week of surgery, and a new periodontal pack was placed in the surgical area if required. Palatal stents and sutures were removed after satisfactory healing in the second week. The patients were instructed to visit in the first, third, and sixth months, respectively, after the surgical procedure. Scaling and polishing were performed at each visit, and all records were taken at each visit.
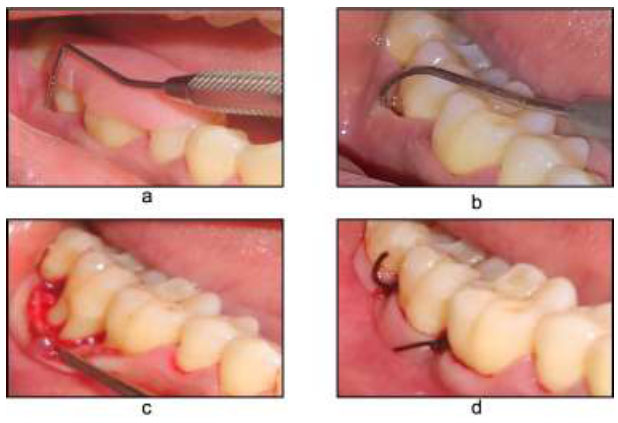
Open flap debridement a) Pre-operative with stent V-PPD, b) HPD, c) Flap reflection, d) Flap sutured.
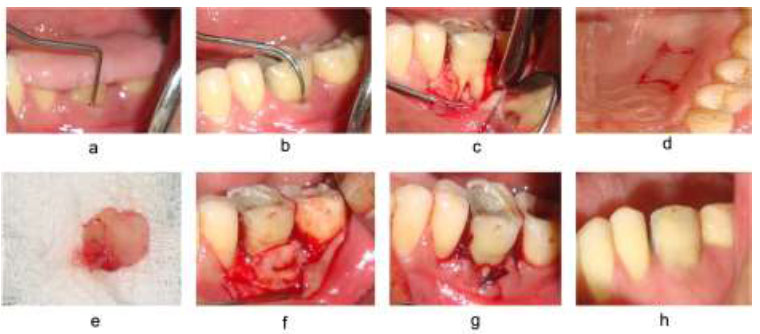
Autogenous periosteal graft with open flap debridement a) Pre-operative with stent V-PPD, b) HPD, c) Flap reflection, d) Incision at the donor site, e) APG harvested, f) APG placed at the recipient site, g) Flap sutured, h) post-operative at 6 months.
2.8. Statistical Analysis
IBM SPSS software, version 27 (IBM Co., Armonk, NY, USA), was used to conduct all statistical analyses. The normality test was performed with the Shapiro–Wilk test. Descriptive statistics were performed for V-PPD, HPD, RGM, L, and V-RAL. A comparison between baseline and 3 months and 6 months was made using the paired t-test. An independent t-test was carried out to compare the control and test groups from baseline to 3 months and from baseline to 6 months. In order to compare the PBI and PI at baseline, 3 months and 6 months, the paired t-test was performed. The outcome was considered highly significant, very significant, significant, and non-significant when p = <0.001, p = <0.01, p = <0.05, and p = >0.05, respectively, at a 95% confidence interval (CI).
3. RESULTS
A total of 24 patients presenting mandibular buccal Class II furcation defects, including 12 males and 12 females with a mean age of 39.90 years, participated in this study. All patients were equally divided into a control group (treated with OFD) and a treatment group (treated with OFD+APG). During the study period, the wound healing was uneventful. Neither any APG was necrosed nor any of the sites removed from the study. None of the included patients dropped out before the completion of the study, and all patients were pleased with the outcome. Shapiro–Wilk test showed that all data were normally distributed; therefore, parametric statistics were performed.
3.1. Clinical Outcomes
The PI and PBI scores were compared at baseline, 3 months, and 6 months in both groups using the paired t-test. Table 2 presents that none of the groups showed any significant changes over the 6 months.
| Group | Parameter | Mean | SD | Mean | SD | MD | p-value |
|---|---|---|---|---|---|---|---|
| - | - | Baseline | 3 Months | - | - | ||
| Control (OFD) | PI | 0.76 | 0.07 | 0.75 | 0.1 | -0.007 | 0.640 |
| PBI | 0.65 | 0.10 | 0.67 | 0.16 | -0.02 | 0.650 | |
| - | Baseline | 6 Months | - | - | |||
| PI | 0.76 | 0.07 | 0.77 | 0.13 | 0.01 | 0.682 | |
| PBI | 0.65 | 0.10 | 0.72 | 0.11 | -0.07 | 0.251 | |
| Treatment (OFD+APG) | - | Baseline | 3 months | - | - | ||
| PI | 0.73 | 0.05 | 0.71 | 0.08 | -0.007 | 0.173 | |
| PBI | 0.65 | 0.16 | 0.65 | 0.16 | -0.02 | 0.900 | |
| - | Baseline | 6 Months | - | - | |||
| PI | 0.73 | 0.05 | 0.70 | 0.10 | 0.03 | 0.146 | |
| PBI | 0.65 | 0.16 | 0.75 | 0.14 | -0.90 | 0.131 | |
The clinical parameters, such as V-RAL, RGML, V-PPD, and HPD, were compared at baseline between the two groups using an independent t-test, which indicated that there was no significant difference in any clinical parameters between the two groups (Table 3). On the other hand, while clinical parameters were compared from baseline to 3 months, all parameters showed a highly significant reduction in both groups (Table 4). Moreover, a comparison from baseline to 6 months also showed a significant reduction in all parameters in both groups except for the RGML parameter in the control group (Table 5). In addition, while comparing the parameters between the two groups, all parameters showed significant differences after 6 months (Table 6).
Complete obliteration of furcation was accomplished in 2 (16.66%) sites in the study group compared to none in the control group. The improvement in horizontal furcation classification from Class II to Class I was seen in 6 (50%) sites in the test group and 2 (16.66%) sites in the control group. However, despite the reduction in hori- zontal probing depth, no change was seen in 10 sites of the control and 4 sites of the test group (Table 7).
| Parameter | Control | Test | MD | p-value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| V-RAL | 9.78 | 1.15 | 10.00 | 1.12 | -0.222 | 0.409 |
| RGML | 5.08 | 0.80 | 5.30 | 0.78 | -0.222 | 0.240 |
| V-PPD | 4.63 | 0.93 | 4.75 | 0.99 | -0.111 | 0.626 |
| HPD | 7.91 | 0.99 | 7.67 | 1.07 | -0.250 | 0.560 |
| Group | Parameter | Baseline | 3 Months | MD | p-value | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Control (OFD) | V-RAL | 9.77 | 1.15 | 8.72 | 0.94 | 1.06 | 0.0001* |
| RGML | 5.08 | 0.80 | 5.52 | 0.69 | -0.44 | 0.0001* | |
| V-PPD | 4.63 | 0.93 | 3.27 | 0.78 | 1.36 | 0.0001* | |
| HPD | 7.91 | 0.99 | 5.41 | 0.99 | -2.50 | <0.0001* | |
| Test (OFD+APG) | V-RAL | 10.00 | 1.121 | 7.16 | 1.08 | 2.83 | 0.0001* |
| RGML | 5.30 | 0.78 | 4.55 | 0.73 | 0.75 | 0.0001* | |
| V-PPD | 4.75 | 0.99 | 2.58 | 0.96 | 2.16 | 0.0001* | |
| HPD | 7.67 | 1.07 | 5.08 | 1.31 | 2.58 | 0.0001* | |
| Group | Parameter | Baseline | 6 Months | MD | p-value | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Control (OFD) | V-RAL | 9.67 | 1.14 | 8.52 | 0.91 | 1.25 | 0.0001* |
| RGML | 5.08 | 0.80 | 5.39 | 0.83 | -0.30 | 0.062 | |
| V-PPD | 4.63 | 0.93 | 3.19 | 0.82 | 1.44 | 0.0001* | |
| HPD | 7.91 | 0.99 | 5.08 | 0.99 | -2.83 | 0.0001* | |
| Test (OFD+APG) | V-RAL | 10.00 | 1.21 | 6.30 | 1.14 | 3.69 | 0.0001* |
| RGML | 5.30 | 0.78 | 3.94 | 0.75 | 1.36 | 0.0001* | |
| V-PPD | 4.75 | 0.99 | 2.47 | 0.94 | 2.27 | 0.0001* | |
| HPD | 7.67 | 1.07 | 3.42 | 2.02 | 4.25 | 0.0001* | |
| Parameter | Control | Test | MD | P-value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| V-RAL | 1.25 | 0.84 | 3.69 | 1.30 | 2.44 | 0.001* |
| RGML | -0.30 | 0.95 | 1.36 | 0.93 | 1.66 | 0.001* |
| V-PPD | 1.44 | 1.05 | 2.27 | 1.27 | 0.83 | 0.001* |
| HPD | 2.83 | 0.83 | 4.25 | 1.60 | 1.42 | 0.007* |
| Parameter | Control | Test |
|---|---|---|
|
OFD (n=12) No. of Sites (%) |
APG+OFD (n=12) No. of Sites (%) |
|
| Complete closure (HPD=0) | 0 (0%) | 2 (16.66%) |
| Change from Class II to Class I (HPD<3) | 2 (16.66%) | 6 (50%) |
| No change | 10 (83.3%) | 4 (33.33%) |
4. DISCUSSION
The current research aimed to compare the efficiency of APG along with OFD and only OFD in the treatment of Class II furcation defects. The current study assessed and compared the treatment effectiveness over 6 months. No significant differences were observed at baseline among all parameters in both groups. Removing the subgingival calculus and plaque is one of the primary goals of periodontal therapy, which can be easily maintained by patients on a long-term basis. Proper postoperative maintenance and care could arrest the disease progression.
In the present study, OFD was used as a control treatment following different previous clinical studies, which also evaluated the regenerative techniques in the treatment of infra-bony defects [6, 15, 21-28]. While the outcomes were generally inferior concerning the regenerative procedures under study, a significant beneficial effect of treatment was noted. OFD technique is mostly used due to its cost-effectiveness. Moreover, in the case of an infra-bony defect, regenerative surgery is not always considered the first choice.
PI and PBI scores exhibited that healthy gingival conditions and good oral health were maintained throughout the study by all participating patients. The PI score and PBI scores were low at both baseline and at 6 months. Different short-term [26, 32] and long-term data [33, 34], indicated low PI scores, which indicated optimum oral hygiene with appropriate clinical attachment gain. In this study, patients were regularly informed about oral hygiene to keep the PI and PBI low.
In the present study, both APG as a barrier and OFD showed statistically significant improvement in vertical V-PPD and HPD as well as V-RAL throughout the observation period of the study. When the results were compared between the two groups, statistically significant differences were observed for the clinical parameters tested.
Gaining clinical attachment level indicates that clinical regeneration occurs after the furcation therapy [35-37]. Measuring attachment gain in horizontal and vertical directions is a common practice in regeneration furcation treatment [37, 38]. The degree of furcation defects should be reduced in an appropriate amount during the regenerative therapy so that the defects can be maintained by routine oral hygiene procedures [37, 39]. In the current study, vertical relative attachment levels increased significantly in both groups. In the treatment group, the mean V-RAL gain was 3.69 ± 1.30 mm, and in the control group, it was 1.24 ± 0.84 mm at 6 months as compared to the baseline values. When the mean differences in the V-RAL gain were compared, a greater gain of CAL (2.44 mm) was witnessed in the treatment group compared to the control group.
In the present study, the results for the gain in CAL in the control group are comparable to the results reported by previous studies for OFD. Sharma and Pradeep (2011) compared the efficacy of platelet-rich fibrin with OFD in lower class II furcation defects and reported a similar gain in CAL 1.27 ± 0.46 mm in the OFD group [27]. Similar results were also reported by Khanna et al. (2012) and Paul et al. (1992) while treating the lower arch class II furcation using the OFD [40, 41].
In order to minimize local reinfection, the ultimate purpose of any periodontal treatment is to reduce PPD. While a narrow pocket predicts the negative progression of disease in the future, deep pockets indicate the risk of disease progression [42]. In this study, a significant reduction was observed in V-PPD in both groups. However, the treatment group exhibited a significant reduction in V-PPD when compared with the control group after 3 months and 6 months. This outcome supports the results of previous studies [17, 20, 21, 29]. However, a previous study concluded a higher PPD reduction of 4.14±0.92 mm with APG in the management of class II furcation defects [15]. This might be due to the inclusion of variable depths of periodontal pockets.
The current study presented significant improvement in both groups; nevertheless, the treatment group showed more improvement, which corrected from class II furcation defects to class I. A total of 33.33% of patients in the treatment group improved from class II furcation to class I, and 16.66% improved to Class 0. Meanwhile, the control group showed a total of 16.60% defect change from class II to class I and 83% of patients remained in class II. A study on class II furcation defects showed 2 out of 11 defects in the acid + CTG group [17], while Deo et al. (2008) reported the closure of 2 of 10 cases [29]. Although the sample size of the current study and the shorter evaluation period did not conclude that one procedure overweighed the other, it could be assumed that APG, along with OFD, more efficiently closed the furcation class II defect over the OFD-only procedure.
An accurate assessment of the regeneration of the hard tissue could be achieved with the second surgical procedure on the operated site. However, sufficient information on the interface between the root surface and bone is still missing with these procedures, as well as the assessment of new periodontal ligaments and cementum [43]. The only reliable method is a histological evaluation that determines all these components of the attachment apparatus. Therefore, the current study should not assume that the true periodontal regeneration occurred, as no histological experiment was conducted in this study. Previous histologic studies on periodontal wound healing showed a normal repair process of the periodontium after OFD [44, 45]. Healing usually occurs when the junctional epithelium forms over the connective tissue adjacent to the root plane's surface. A limited amount of new cementum and bone form at the base of the defect. Moreover, a minimum extent of coronal growth of alveolar bone has been witnessed, which is not equal to the height of newly formed cementum-linked tissue [46, 47]. However, these histological outcomes could not be compared with the findings of the current study due to the absence of histological experiments.
The current study possessed some limitations. This study included all patients based on the convenient sampling technique; therefore, a small sample size might restrict the analysis of the outcome. In addition, a six-month analysis is not enough to reach a proper conclusion about a study; hence, a long-term analysis is also required to determine the stability of the results and well-controlled studies are needed to confirm the findings of the present study.
CONCLUSION
APG, along with OFD as a barrier in the treatment of class II mandibular furcation defects, showed significant improvement in clinical parameters V-RAL, RGML, HPD, and V-PPD compared to the OFD-only treatment. Therefore, APG should consider a conventional treatment option for periodontal furcation defects.
AUTHORS’ CONTRIBUTION
A.J. and P.B. contributed to the conceptualization of the study, methodology, and investigation. M.S.K. curated the software. M.A. and K.G. carried out the validation. M.A. performed the formal analysis. M.S.K. and M.A. contributed to the resources. A.J. performed the data curation. K.G. and M.A. wrote the original draft. K.G. and M.S.K. contributed to the writing, review, and editing. P.B. carried out the supervision. All authors have read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| APG | = Autogenous Periosteal Graft |
| OFD | = Open Flap Debridement |
| GTR | = Guided Tissue Regeneration |
| PBI | = Papillary Bleeding Index |
| RGML | = Relative Gingival Marginal Level |
| V-RAL | = Vertical Relative Attachment Level |
| V-PPD | = Vertical Probing Pocket Depth |
| HPD | = Horizontal Probing Depth |
| CTGs | = Connective Tissue Grafts |
| GTR | = Guided Tissue Regeneration |
| CI | = Confidence Interval |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Review Board (IEC/MDCRC/2011-12/0037) of Modern Dental College and Research Center, Indore, India.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.


