All published articles of this journal are available on ScienceDirect.
Effect of Fluorine Addition on the Topographical Changes of the Dentine Tubular Walls and the Percentage of Fluorapatite in the PILP System
Abstract
Aims
This study aimed to determine the effect of fluorine in dentin remineralization. Guided tissue remineralization has been shown to remineralize affected dentin by forming intrafibrillar and extrafibrillar minerals. Through polymer-induced liquid precursor system, crystals are formed with small sizes and occur intrafibrillarly. The addition of fluorine can form larger fluoroapatite crystals and complete the remineralization to the extrafibrillar. Existing research only focuses on the dentine surface, while there has been no research to prove remineralization in the dentine tubular walls.
Objectives
The objective of this study was to determine the effect of adding 5ppm and 25ppm fluorine in the polymer-induced liquid precursor system on topographical changes and the percentage of fluoroapatite of the dentinal tubule walls.
Methods
Demineralized dentin blocks were immersed in 5ppm and 25ppm fluorine remineralization solution. Dentine blocks were cross-sectioned using fracture method and analyzed using field emission – scanning electron microscope, and x-ray diffraction.
Results
Topographical changes occurred on the dentinal tubule walls after remineralization with the addition of 5ppm and 25ppm fluorine through field emission scanning electron microscope test. Statistical tests were performed using SPSS 25 (SPSS inc) software to analyze the X-ray diffraction data. X-ray diffraction analysis showed that there was no statistically significant difference between the addition of fluorine to the percentage of fluoroapatite mineral phase, but substantially there was an increase in the percentage of fluorapatite.
Conclusion
The addition of fluorine in the polymer-induced liquid precursor system does not influence changes in the topography of the dentinal tubule walls and the percentage of fluoroapatite.
1. INTRODUCTION
The remineralization that occurs in dentin is different from enamel. Initially, the concept of remineralization was conventional or Top-Down remineralization that required residual mineral crystals [1-3]. Currently, another strategy is being developed, which is Guided Tissue Remineralization (GTR) or Bottom-Up [1, 2]. The GTR principle uses nanotechnology and biomimetic materials to achieve intrafibrillar remineralization and extrafibrillar in dentin collagen [3-5].
One of the GTR methods is the Polymer-Induced Liquid Precursor (PILP) system. Gower et al. (2000) used a polyanionic polymer that can coat nanoprecursors to form a nanoprecursor liquid [6, 7]. Burwell et al. (2012), the PILP system with polyaspartic acid was proven to be able to remineralize dentin collagen intrafibrils on the 14th day by Transmission Electron Microscopy (TEM) evaluation [8]. Saxena et al. (2018) proved that the addition of fluorine in the PILP system was able to remineralize rat tail tendons (composed of 97% type I collagen) intrafibrillarly and extrafibrillarly [9]. This research was then continued by Thysa, Clarissa, and Muslim on the block surface dentin. Clarissa et al. (2019), through TEM tests, proved that intrafibrillar and extrafibrillar remineralization was proven to occur with the densest apatite crystal content when 5ppm fluorine was added [10]. Existing research has proven that the addition of fluoride to the PILP remineralization solution can influence changes in the diameter of dentin tubules, the intertubular thickness of dentin, as well as observing mineral precipitation on the dentin surface, but the phenomenon of remineralization on the walls of dentin tubules has never been studied [9, 11].
The PILP system uses bioactive materials, such as Casein Phosphopeptide Amorphous Calcium Phosphate (CPP-ACP), fluorine, calcium silicate, and bioactive glass, as well as polycarboxylic acid-based polymers as NCP analogs [8, 11]. Based on several studies, the size of the molecules formed in the PILP system is stabilized at the nano size so that extra fibrillar remineralization becomes difficult [8, 9, 11]. Fluorine is one of the bioactive materials that can be added to the PILP system to obtain fluoroapatite crystals with larger molecular size, stability, and more resistance to acids than hydroxyapatite crystals [12, 13]. The addition of fluorine in the remineralization of the PILP system is expected to form intrafibrillar and extrafibrillar remineralization so that it can restore the mechanical properties of demineralized dentin [9].
The walls of the dentin tubules have an important role in the adhesive system of a restoration material [14, 15]. Dentin tubules are the connection between the pulp tissue and the Dentino Enamel Junction (DEJ) and make up the bulk of the dentin. The walls of the dentin tubules influence the surface area, which plays a role in the bond between the adhesive system and dentin. In the adhesive system, the bonding will be in direct contact with the walls of the dentin tubules so that remineralization of the dentin tubules will strengthen the collagen structure and produce a stronger bond between the bonding and the tooth structure [14, 15]. A strong collagen structure will reduce the risk of restoration microleakage, thus resulting in a more durable restoration. Existing research has proven that the addition of fluoride to the PILP remineralization solution can influence changes in the diameter of dentin tubules, the intertubular thickness of dentin, as well as observing mineral precipitation on the dentin surface, but the phenomenon of remineralization on the walls of dentin tubules has never been studied.
By adding fluorine to the PILP system, fluoroapatite crystals are expected to not only form on the dentin surface but also to penetrate and remineralize the dentin tubule walls. To strengthen the evidence for the success of adding fluorine in the remineralization of dentin tubule walls, in vitro research was carried out on dentin block samples that were soaked in a remineralization solution with a fluorine concentration of 5 ppm and 25 ppm for 14 days. The samples were cross-sectioned, then the dentin tubules were observed using the Field Emission – Scanning Electron Microscope (FE-SEM) test, and the percentage of the fluoroapatite mineral phase was analyzed using the X-Ray Diffraction (XRD) test.
2. MATERIALS AND METHODS
This in vitro study was approved by the Dental Research Ethics Committee, with ethical clearance no: 08/Ethical Exempted/FKGUI/III/2023. A total of 20 extracted human maxillary premolars without caries and restoration were selected as research samples [8]. The teeth were cleaned to remove soft tissue remnants and debris and were then placed in deionized water with thymol and stored in a refrigerator at 4°C for a maximum of 14 days before experimental treatment [8].
The experimental model described by Burwell was used in this study with modification [8]. Dentin blocks cut from the mid-coronal region of the teeth perpendicular to the direction of the tubules [8]. Dentin blocks were shaped in each tooth sample with a length of 7mm and width of 5mm, and a thickness of 3mm [8]. All samples were measured uniformly and calculated properly using a digital caliper.
The surface of each sample was roughened with SiC abrasive paper to 320–1200 grit (Nikken / Nihon Kenshi #2000, Japan) and then polished [8]. The entire surface of the sample was coated with varnish nail polish (Maybelline, New York, USA) to prevent demineralization, except for an exposed area of 7x5mm. After artificial caries lesions were formed, the samples were rinsed with aquabidest and immersed in a remineralization solution at a temperature of 37 °C, except for the control group [8].
PILP remineralization solution (Biochemistry Labora- torium, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia) was prepared using the following steps: 50 mM tris buffered solution was added with 0.9% NaCl and 0.02% NaN3 [8]. The solution was divided into two halves [8] To the first solution, 9 mM CaCl2 was added, and 4.2 mM K2HPO4 and NaF were added to the second solution [8]. The solutions were filtered with 0.22 μm filter paper [8]. Polyapartic acid was added to the CaCl2 solution and stirred for five minutes [8]. The two solutions were then mixed and stirred for five minutes before use. Subsequently, the solution was divided into three concentrations (5ppm, 25ppm, and no fluorine). The sample was then divided into five groups: Group I comprised normal dentin as a control, Group II underwent demineralization without remineralization, Group III underwent remineralization without addition of fluorine in the PILP solution for 14 days, Group IV underwent remineralization with addition of 5ppm fluorine in the PILP solution for 14 days, and Group V underwent remineralization with addition of 25ppm fluorine in the PILP solution for 14 days. All samples were stored in an incubator (Shanghai Zhichu Instrument Co., Ltd, Shanghai, China) with continuous shaking at 37 °C, except for the first group [8].
Samples were fractured horizontally to get the cross-section surface and analyzed with FE-SEM (Ultra-high Resolution Scanning Electron Microscope SU9000II, New York, USA) to observe the topographical changes of the dentine tubular walls and with XRD (D6 Phraser Bruker, New York, USA) to get the percentage of fluoroapatite. Statistical tests were performed using SPSS 25 (SPSS inc., Chicago, Illinois) software to analyze the XRD data. The data were analyzed using the normality distribution test in each group using Shapiro–Wilk statistics because the sample size was less than 50. The differences between the four groups were analyzed using a one-way ANOVA test to determine if the data distribution was normal and the Kruskal-Wallis test to determine if the data distribution was not normal. Statistical analysis using the SPSS 25 software employed a significance level of <0.05.
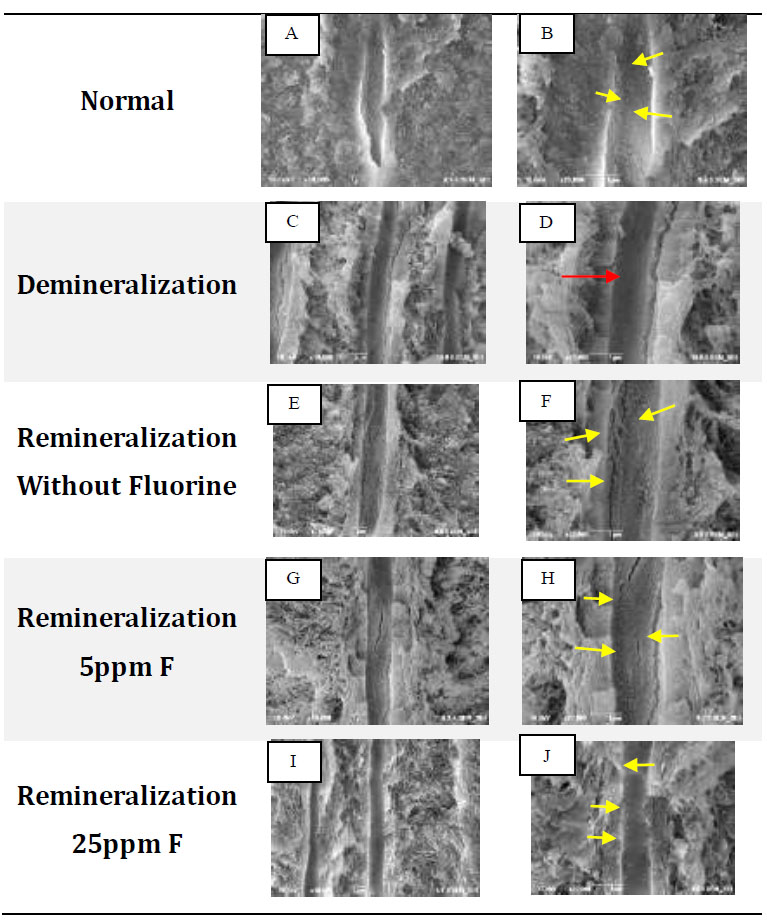
FE-SEM test results: A, C, E, G, I with 10,000x magnification, B, D, F, H, J with 20,000x magnification. Yellow arrows show differences in topography and characteristic white features found on the walls of dentinal tubules. Red arrows indicate the topography of the empty, flat walls of dentinal tubules.
3. RESULTS
In normal dentin, an uneven topography of the dentin tubule walls can be seen in the form of a white image with irregular edges spread along the tubule walls (Fig. 1A, B). In the demineralization group, the wall topography was visible, which was flat, smooth, concave, and looked dark or empty without any white appearance (Fig. 1D). In the remineralization group without fluorine, a white image with regular borders was visible, and evenly distributed along the dentin tubules (Fig. 1F). In contrast to the group without fluoride, with 5 ppm fluorine remineralization, a white image with regular edges with an uneven topography on the dentin tubule walls was visible (Fig. 1H). In Fig. (1J), the dentin tubules appear to be filled and the distance between the particles was so close that the particle edges were difficult to distinguish. Based on FE-SEM analysis, the five groups provided different topographic results.
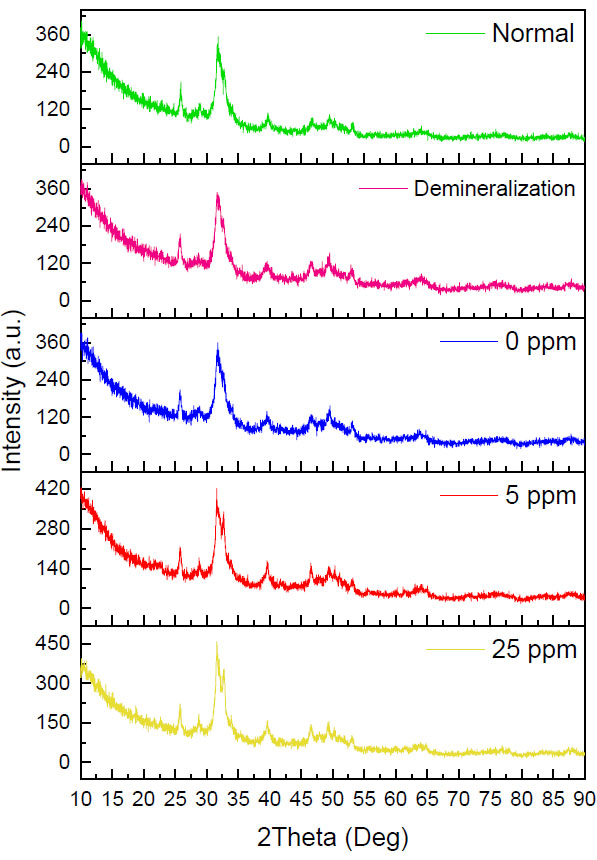
Diffractogram for each treatment group. HA: Hydroxyapatite, FA: Fluoroapatite.
| Groups | Fluoroapatite Mineral Phase Percentage (%) | |
|---|---|---|
| Median (Min-Max) | P value | |
| Normal | 20 (8-34) | 0.815 |
| Demineralization | 18 (17-25) | |
| Remineralization without fluorine | 20 (17-28) | |
| Remineralization 5ppm F | 23 (23-35) | |
| Remineralization 25ppm F | 24 (20-27) | |
In Fig. (2), the five groups have the same pattern, the presence of a narrow peak with high intensity at 2ϴ (31o) and additional peaks in the 5ppm and 25ppm fluorine remineralization groups which are located at 2ϴ (32o). The peak in the demineralization group looks wider when compared to the other four groups, so the diffractogram looks more sloping. In contrast to the 5ppm and 25ppm fluorine remineralization groups, the peaks looked very narrow with high intensity in the 5ppm and 25ppm fluorine remineralization groups. The red arrow in Fig. (2) shows the hydroxyapatite and fluoroapatite peaks, which look very close together and difficult to distinguish. However, the fluoroapatite peaks in the 5ppm and 25ppm remineralization groups are clearly visible and separated from the hydroxyapatite peak.
The normality distribution test showed the distribution data was not normal; therefore, the test was continued with the Kruskal Wallis test. Kruskal Wallis test results can be seen in Table 1. The Kruskal Wallis test showed a result of p=0.815. Although there was no statistically significant difference, there was a substantial increase in the percentage of fluoroapatite mineral phase in the 5ppm and 25ppm remineralization groups when compared with normal and demineralized dentin. In the FE-SEM test results, topographic changes in the dentin tubule walls prove that remineralization occurs in the dentin tubule walls. Apart from that, the crystals formed from remineralization on the dentin tubule walls were also confirmed through XRD testing, and it was found that there was an increase in the percentage of fluoroapatite mineral phase in the remineralization group with the addition of fluorine.
4. DISCUSSION
In Fig. (1B and 1D), the edges of the crystals are irregular and appear faint or not very clear. This shows that the density is not too high in the hydroxyapatite crystals found in normal dentin [16]. Hydroxyapatite crystals are evenly distributed and not stacked. Ferraz et al. (2004) stated that the hydroxyapatite nanocrystals have an appearance like spherical or round lumps with irregular edges [17]. The inorganic content of normal dentin is mostly hydroxyapatite crystals [16]. In Fig. (1D), the tubule walls look darker with a flat topography. This shows that the tubules look empty without any crystals. The image of empty dentin tubules proves that the demineralization solution used in this study can dissolve the hydroxyapatite crystals found on the walls of dentin tubules [18, 19]. The results of the FE-SEM test for the remineralization group without fluoride showed that remineralization was proven to occur, which was characterized by the refilling of the dentin tubules with white irregular and regular edges along the dentin tubules (Fig. 1F). The white appearance with irregular edges resembles hydroxyapatite crystals, while the white rounded appearance, larger size, and clear and regular edges are considered fluoroapatite crystals [13, 17, 20, 21]. Based on Kim's research, the SEM results of ACP nanoparticles stabilized by pAsp show that the nanoparticles are amorphous and spherical in shape, which form clusters and are distributed homogeneously [22].
In the 5ppm remineralization group (Fig. 1H), the white image was considered fluoroapatite crystals which had changed from hydroxyapatite due to the addition of fluorine ions. Based on the research by Sumi et al., the addition of fluoride at a low concentration (0-8 ppm) to a solution containing calcium and phosphate ions will modify the topography of the hydroxyapatite crystal structure [11]. The precipitation of fluoride will slowly change into a needle-like material that grows on the surface of hydroxyapatite crystal seeds, then develops into long rod-like crystallites, and finally, they arrange themselves regularly to form a hexagonal structure [12, 21, 22].
To confirm the percentage of crystals formed, the percentage of the fluoroapatite mineral phase was analyzed via an XRD test. The data in the diffractogram were matched with the database, and it was found that the dominant crystal type was hydroxyapatite in the five groups (Fig. 2). This is in line with the FE-SEM test results in Fig. (1B), where it is suspected that the white image in the normal dentin group is hydroxyapatite crystals, so it can be confirmed that in the normal group, the dentin tubules are dominated by hydroxyapatite crystals [12, 13, 20, 23].
Fig. (2) confirms the formation of fluoroapatite, which is characterized by the presence of separate FA peaks at 2ϴ (32o). This is in line with the FE-SEM test results in Fig. (1H), where the characteristics of most of the crystals resemble fluoroapatite crystals. With a concentration of 5ppm, the remineralization solution still has sufficient time to stabilize the size of the ACFP nanoprecursor so that it can infiltrate and induce intrafibrillar reminera- lization and can form larger crystals between collagen fibers [3, 5, 8, 11]. The addition of fluorine at a low concentration can increase the crystal size, but crystallization still occurs slowly, sofluoroapatite still has time to form regularly in the extrafibrillar [9].
The diffractogram pattern in the 25ppm reminera- lization group also showed the addition of a fluoroapatite peak (Fig. 2). In the results of the FE-SEM image, the walls of the dentin tubules were covered with dense particles which were considered precipitation of calcium and phosphate minerals which did not have time to stabilize, thus forming crystallization on the surface of the dentin tubule walls. Fluoroapatite crystals were considered more likely to be formed extrafibrillarly because the high concentration of fluoride ions means that the crystals can only be stabilized in a short time [9, 23]. There is not enough polymer in the remineralization solution. Hence, the crystals quickly form on the dentin surface and close the entrance for the precursors to enter, penetrate, and crystallize intrafibrillarly [9, 11].
As shown in Table 1, the insignificant research results were thought to be caused by the remineralization solution consisting of weak acids so that only a small amount of crystal content in the demineralization group was dissolved. The use of acetic acid buffer pH 5 is intended to form artificial caries that resemble the affected dentin so that the inorganic content in it is still high enough to prevent total degradation so that the artificial caries that are formed can still be remineralized [18, 19, 24].
Results that are not significantly different are also thought to be influenced by the type and number of samples. Using other types of samples, such as teeth with real caries, might affect the significance of the results of this study because teeth with caries have real dentin-affected conditions and will provide representative results. Apart from that, increasing the sample size can also be considered to influence the data distribution and is expected to increase the significance of the research results.
Although not statistically significantly different, the median, minimum, and maximum values obtained in the remineralization group showed an increase in the percentage of fluoroapatite along with an increase in the percentage of fluorine. Based on this analysis, it can be said that the addition of fluoride has been proven to influence the remineralization of the dentin tubule walls, which is characterized by changes in topography and an increase in the percentage of fluoroapatite in the dentin tubule walls. Additionally, it should be considered that other remineralizing agents have been introduced, such as biomimetic hydroxyapatite, casein phosphopeptide- amorphous calcium phosphate, and calcium sodium phosphosilicate [25-27]. These compounds showed promising results and could be combined with Fluorine in the PILP System in future research to understand their mutual effects [25, 26].
The use of the XRD test to confirm the fluoroapatite and hydroxyapatite content in samples has several limitations, including the difficulty of distinguishing fluoroapatite and hydroxyapatite because the crystalline structure is almost the same. The relatively narrow sample surface area and the difficulty of obtaining a flat sample surface using the fracturing method provide background noise, which can complicate the analysis of sample test results. Some of the limitations above can be minimized by preparing specimens with uniform sizes and flat surfaces, as well as communicating with the laboratory regarding the desired test points so that the data becomes more representative [27].
This research proves that the addition of fluoride in the remineralization solution in PILP can penetrate the dentin tubules and form extrafibrillar remineralization, but intrafibrillar remineralization cannot be proven visually. Previous research has proven the occurrence of intra- fibrillar remineralization using the TEM test; however, the test was carried out on the dentin surface, which was in direct contact with the remineralization solution, but the test was not carried out on the dentin tubules. Therefore, it cannot be proven that the addition of fluorine to the remineralization solution using the PILP system can penetrate and form intrafibrillar remineralization in dentin tubules, so a TEM test is needed on cross-sectioned dentin blocks to strengthen the results of this study.
CONCLUSION
The addition of fluorine concentrations of 5ppm and 25ppm in the PILP system does not cause noticeable topographic changes in the dentin tubule walls in the form of the formation of fluoroapatite crystals. Fluoride also does not increase the percentage of fluorapatite mineral phase in dentin tubules significantly. A high fluorine concentration can cause rapid precipitation of minerals that are formed on the dentin surface and inhibit the penetration of the remineralization solution into the dentin tubules.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ACFP | = Amorphous Calcium Fluoride Phosphate |
| FE-SEM | = Field Emission – Scanning Electron Microscope |
| GTR | = Guided Tissue Remineralization |
| pAsp | = Poliaspartic Acid |
| PILP | = Polymer-Induced Liquid Precursor |
| TEM | = Transmission Electron Microscopy |
| XRD | = X-Ray Diffraction |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Dental Research Ethics Committee of Universitas Indonesia, Jakarta, with ethical clearance no: 08/Ethical Exempted/FKGUI/III/2023.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
This research was funded by Universitas Indonesia for the implementation of the Hibah Publikasi Terindeks Internasional (PUTI) postgraduate program 2023-2024 with agreement letter no: NKB-193/UN2.RST/HKP. 05.00/2023.
ACKNOWLEDGEMENTS
Declared none.


