All published articles of this journal are available on ScienceDirect.
The Effect of Various Water-soluble Chitosan Concentrations on Physical Properties and Antibiofilm Ability of Mineral Trioxide Aggregate
Abstract
Objective
Using Mineral Trioxide Aggregate (MTA) has several challenges as an apex closure material, such as a long hardening time, making it susceptible to dissolution and being washed away by blood flow, a gritty consistency that is difficult to manipulate, and low compression resistance. In addition, the antibacterial properties of MTA against E. faecalis, the bacteria persisting in periapical lesions, have shown controversial results. This study aimed to analyze the effect of adding various concentrations of water-soluble chitosan to MTA on the setting time, compression strength, and antibiofilm ability of E. faecalis.
Materials and Methods
There were three groups in this study, namely, MTA without water-soluble chitosan (MTA), MTA with 5% (MTA-CW5), and MTA with 10% (MTA-CW10) water-soluble chitosan. 0.5 g MTA powder (ProRoot MTA, Dentsply Tulsa Dental, Tulsa, OK, USA) was stirred in the MTA group with 0.166 ml of distilled water. In the MTA-CW5 and MTA-CW10 groups, 40 µl of 5% and 10% water-soluble chitosan was added to the mixture of 0.5 g MTA powder and 0.166 ml distilled water. Then, the three groups were tested for setting time, compression strength, and antibiofilm activity against E. faecalis. Setting time and compression strength values were analyzed by parametric statistics (ANOVA). Bacterial cell viability values on a numerical scale were statistically analyzed using the Kruskal–Wallis non-parametric analysis. The level of significance used was p < 0.05.
Results
There was a significant difference in setting time among the three groups, from the fastest to the slowest setting time, respectively: MTA-CW5, MTA, and MTA-CW10. The same thing happened in the compression strength test, with the lowest mean value shown in the MTA group at 50.53 + 6.18 MPa. The viability of E. faecalis between the MTA and MTA-CW5 groups did not have a statistically significant difference.
Conclusion
The setting time of MTA with 5% water-soluble chitosan was fast, but adding 10% water-soluble chitosan slowed the setting time of MTA. Meanwhile, increased water-soluble chitosan concentration led to increased compression strength and antibiofilm activity of MTA against E. faecalis.
1. INTRODUCTION
Mineral Trioxide Aggregate (MTA) has been used in various endodontic procedures, such as perforation repair, pulp capping, apexification, apexogenesis, endodontic surgery, and pulp regeneration [1]. This material is a good sealant, hydrophilic, biocompatible, hard tissue stimulant, and antibacterial [2-4]. To date, MTA is still believed to be the gold standard of apex sealant and perforation repair material, as it has hydrophilic and antibacterial properties [1].
Using MTA has several challenges as an apex closure material, such as a long setting time (3–4 hours), making it susceptible to dissolution and being washed away by blood flow, a gritty consistency that is difficult to manipulate, and low compression resistance [5-7]. In addition, the antibacterial properties of MTA against Enterococcus faecalis, the bacteria persisting in periapical lesions, have shown controversial results. Studies by Koruyucu et al. (2015) and Liu et al. (2020) stated that MTA showed antibacterial activity against E. faecalis [8, 9]. Research by Kim et al. (2015) reported that ProRoot MTA and MTA-Angelus could not inhibit the development of E. faecalis. This agrees with the results of the studies by Estrella et al. (2000) and Torabinejad et al. (1995) [5, 10]. Synthetic and natural polymers have been investigated as additive materials in MTA and are known to improve physical properties and material manipulation [11, 12].
Chitosan is a natural polymer with antibacterial, bioactivity, biocompatibility, biodegradability, and non- toxic properties [13, 14]. However, the application of chitosan is limited due to its low solubility at neutral pH [15, 16]. Research by Panahi et al. (2017) showed the synergistic effect between 2% (v/v) acid-soluble chitosan and dicalcium phosphate on tricalcium silicate-based nanocomposites, which can accelerate the setting time but reduce the compression strength [17]. Research by Subhi et al. (2020) showed that adding 0.625%, 1.25%, and 2.5% (w/v) acid-soluble chitosan resulted in longer setting time and decreased compression strength [18]. In addition, the antibacterial effect of the polysaccharide chitosan has been known to be weak in a neutral pH environment [19].
Chemical modification and molecular weight degradation of chitosan produce water-soluble chitosan derivatives with low viscosity and molecular weight characteristics. Water-soluble chitosan is known to have a superior antibacterial effect, bioactivity, and biocompatibility in dentistry [20, 21]. Research by Wang et al. (2014) showed the potential of oligosaccharide water-soluble chitosan in improving the physical characteristics and accelerating the setting time of bone cement [22]. The study by Chen et al. (2012) showed that the antibacterial activity of water-soluble chitosan against various dental pathogens is more than that of acid-soluble chitosan [23]. The effect of adding water-soluble chitosan to MTA cement remains of interest to researchers. The null hypothesis was that there would be no significant difference in adding water-soluble chitosan to MTA in setting time, compression strength, and antibiofilm ability against E. faecalis. This study aimed to analyze the effect of adding various concentrations of water-soluble chitosan to MTA on setting time, compression strength, and antibiofilm activity against E. faecalis.
2. MATERIALS AND METHODS
2.1. Specimen Preparation
A preparation of 5% and 10% concentration chitosan solution was conducted by dissolving 0.05 g and 0.1 g of water-soluble chitosan powder (Chitosan Wsp, PUI Chitosan and Advanced Materials, University of North Sumatra, Indonesia), respectively, in 1 ml of distilled water, which had been disinfected with UV light for 15 minutes, then vortexed. This study includes three treatment groups: MTA without water-soluble chitosan (MTA), MTA with 5% (MTA-CW5), and MTA with 10% (MTA-CW10) water-soluble chitosan. 0.5 g of MTA powder (ProRoot MTA, Dentsply Tulsa Dental, Tulsa, OK, USA) was mixed with 0.166 ml of distilled water in the MTA group. In the MTA-CW5 and MTA-CW10 groups, 40 µl (based on a pilot study) of 5% and 10% water-soluble chitosan was added to the mixture of 0.5 g MTA powder and 0.166 ml distilled water (Table 1). The specimen size for setting time and compression strength examinations were determined using G*Power analyses.
2.2. Setting Time
The setting time test utilized the methods established by Ber et al. (2007) and Tilakchand, et al. (2021) [15, 24]. The Vicat needle test apparatus was chosen as it is a hydraulic cement setting time test apparatus based on ASTM C 191 [25]. Each specimen was manipulated and placed inside a 10 × 2 mm (diameter × height) acrylic mold for three minutes, as per the working time instructions. The specimens were then placed in an incubator at 37°C with 100% humidity. Setting time tests were performed by one operator using a 300-g Vicat needle with a diameter of 1.0 ± 0.02 mm, starting when the powder came into contact with the liquid. Indentation checks were conducted at the 150th minute, followed by intervals of 30, 15, 10, 5, and 1 minute until there were no indentations in the cement.
2.3. Compression Strength
Compression strength testing was adapted from the research of Jang et al. (2018), which is based on ISO 9917-1 [12]. Specimens were molded in 4 × 6 mm (diameter × height) cylinders. The specimens were incubated for four days at 37°C, with a pH of 7.4 and 100% humidity [12, 26]. After four days, all specimens were polished using 1,200 grit SiC abrasive paper to remove the rough surface. The diameter and height of the specimens were measured using digital calipers. The material’s compression strength was measured with a Universal testing machine (Shimadzu, AGS-5kNX, Japan) with a dropping load speed of 0.5 mm/min.
2.4. Antibiofilm Ability against E. faecalis
E. faecalis ATCC 29,212 stock was cultured on BHI (brain heart infusion) agar, and then the growing colonies were taken to prepare the E. faecalis mother liquor. The suspension of E. faecalis ATCC 29212 was diluted serially to determine the concentration. The bacterial concen- tration used in this study was 2.2 × 105 CFU. Then E. faecalis biofilm was made by putting 100 μl of the bacterial suspension into 96 well-plates according to the research design, then incubated for 24 hours at 37°C. Next, the supernatant was discarded, and the well plate was rinsed with PBS (phosphate buffered saline) to remove planktonic bacteria.
| Specimens | Composition Description | Mixing Methods |
|---|---|---|
| MTA | Calcium silicate-based material ProRoot MTA® (Dentsply Tulsa Dental, Tulsa, OK, USA). LOT number: 0000295845 | Based on manufacture direction use: W/P = 3/1. It consists of 0.5 gr MTA powder and 0.166 ml liquid. |
| MTA-CW5 | MTA ProRoot® (Dentsply Tulsa Dental, Tulsa, OK, USA) with 5% water-soluble chitosan (Chitosan Wsp, PUI Chitosan and Advanced Materials, University of North Sumatra, Indonesia). | Mixing 5% water-soluble chitosan (0.05 gr chitosan powder in 1 ml liquid) into MTA specimen. |
| MTA-CW10 | MTA ProRoot® (Dentsply Tulsa Dental, Tulsa, OK, USA) with 5% water-soluble chitosan (Chitosan Wsp, PUI Chitosan and Advanced Materials, University of North Sumatra, Indonesia). | Mixing 10% water-soluble chitosan (0.1 gr chitosan powder in 1 ml liquid) into MTA specimen. |
The homogeneously mixed cement was placed in a 12 × 2-mm mold (diameter × height) and then allowed to harden for 24 hours at 37°C. After hardening, the cement was ground, and then the ground powder was put into a 15 ml tube. A total of 2.42 ml of BHI (according to the standard ratio of surface area and volume of extracted liquid 1.25 cm2/ml ISO 10993-12) was put into the tube, then incubated for 24 hours (37°C, 5% CO2/air) according to ISO Standards 10993-5. After 24 hours, the extract was taken using a pipette and filtered using a sterile 0.22-µm syringe filter.
Bacterial biofilms formed in each well were added to 100 µl of MTA, MTA-CW5, and MTA-CW10 extraction solutions. In the biofilm-negative control group without treatment, 100 µl of BHI was put into the well. Biofilms exposed to the test materials wereincubated at 37°C for 24 hours. MTT (methylthiazol tetrazolium) test was used to measure the viability of E. faecalis biofilms after exposure to specimens’ cement extracts. Before the MTT test, the plate was rinsed with 100 µl of PBS solution. Then, 100 µl of 5 mg/ml MTT solution was added to each well containing the test material. Next, the plate was incubated for three hours at 37°C. After that, 100 µl of acidified isopropanol was added to each well. The well plate was placed on a shaker for one hour [27, 28]. The optical density value was read on a spectrophotometer (ELISA Reader) with a wavelength of 490 nm. Then, the viability of the bacterial biofilm was calculated using the viability formula:
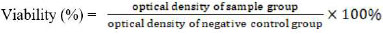 |
2.5. Statistical Analysis
Statistical analysis was performed with SPSS Statistics 25.0 (IBM Software, Armonk, US). Setting time and compression strength values were analyzed using parametric statistics (ANOVA). The significance of each group was analyzed by Bonferroni’s post-hoc test with a significance level of p < 0.05. Bacterial cell viability values were statistically analyzed using the Kruskal–Wallis non-parametric analysis, followed by the post-hoc Mann– Whitney test, with a significance level of p < 0.05.
3. RESULTS
The descriptive statistics of setting time and compression strength of the three treatment groups are presented in Table 2. The results showed the fastest setting time in MTA-CW5 with a mean of 247.2 ± 11.6 minutes and the slowest in MTA-CW10 with a mean of 301.8 ± 14.5 minutes. Compared with the MTA group, MTA-CW5 showed a decrease in setting time, while the MTA-CW10 group experienced an increase in setting time. Bonferroni’s post-hoc Test showed a significant difference in setting time between MTA-CW5 and MTA, MTA-CW10 and MTA, and MTA-CW5 and MTA-CW10 (p < 0.05).
| Variables | Descriptive Statistics | p-value | ||
|---|---|---|---|---|
| MTA | MTA-CW5 | MTA-CW10 | ||
| Setting time | 271.5 ± 14.3 | 247.2 ± 11.6 | 301.8 ± 14.5 | 0.001* |
| Compression strength | 50.53 ± 6.18 | 63.42 ± 3.36 | 77.91 ± 4.47 | |
| Variables | Descriptive Statistics | p-value |
|---|---|---|
| MTA | 11.89 (10.99–14.60) | 0.000* |
| MTA-CW5 | 10.69 (9.64–14.60) | |
| MTA-CW10 | 9.48 (9.18–9.64) |
The highest mean compression strength value was found in the MTA- CW10 group at 77.91 ± 4.47 MPa. In contrast, the lowest mean value of compression strength was found in the MTA cement group at 50.53 ± 6.18 MPa. Bonferroni’s post-hoc Test showed significant differences in compression strength between the MTA-CW5 group with MTA, MTA-CW10 group with MTA, and MTA-CW5 with MTA-CW10 (p < 0.05). Table 3 shows the value of bacterial viability (%) in all groups. Accordingly, the middle value of E. faecalis biofilm viability was in the MTA-CW10, MTA-CW5, and MTA groups (low to high). The viability value between the MTA and MTA-CW5 groups had no statistically significant difference (p ≥ 0.05). Meanwhile, the viability value of E. faecalis between the MTA group with MTA-CW10 and MTA-CW5 with MTA-CW10 had a significant difference (p < 0.05).
4. DISCUSSION
MTA (ProRoot MTA, Dentsply Tulsa Dental, Tulsa, OK, USA) was chosen for its pure content and no additives other than the radio-opacifier, bismuth oxide [29]. The hydration process of MTA contains many microscopic globular air voids, forming a porous solid [30]. These structures are expected to be filled by chitosan, thus improving MTA’s physical and biological properties. Some of the factors influencing this study’s results in the material side of the MTA include powder-to-liquid ratio, pH, temperature, storage medium, storage conditions, and condensation pressure [16, 31-34]. In the present study, the powder-to-liquid ratio was controlled by weighing the powder composition and measuring the liquid phase with a calibrated micropipette. The temperature and environ- mental conditioning of the cement was conductedby placing the specimen in an incubator.
Water-soluble chitosan was chosen because it is more advantageous in the hydration reaction of MTA than acid-soluble chitosan. This is because environmental conditions with low pH can affect the formation of MTA crystals in the hydration phase and lead to weak MTA structure [3]. In addition, acid-soluble chitosan’s antibacterial effect was weak in a neutral pH environment. Thus, derivatization of polysaccharide chitosan to improve solubility in aqueous media with neutral pH was conducted to expand its applications. According to the results of a study by Chen et al. (2012), water-soluble chitosan showed broader antibacterial activity than acid-soluble chitosan [14, 19]. However, the molecular weight of the water-soluble chitosan used in this study is unknown due to the limited availability of test equipment.
The setting time of MTA-CW5 was significantly faster than that of MTA and MTA-CW10. Similar results were stated by Wang et al. (2014), who examined the physical and biological properties of calcium silicate bone cement with various concentrations of Chitosan Oligosaccharide (COS). Adding 2.5% and 5% chitosan was found to shorten the setting time [22]. Panahi et al. (2017) examined the synergistic effect of dicalcium phosphate and chitosan in cement comprising various Portland cement, bismuth oxide, dicalcium concentration phosphate, and 2% chitosan dissolved in acetic acid. Setting time acceleration may be influenced by short-chain chitosan that can bind to water, thus reducing the water in the cement paste and promoting the hydration rate [17]. Lin et al. (2010) also stated that adding inorganic salts can accelerate the setting time. Adding chitosan to MTA facilitates the interaction of MTA metal ions and chitosan amine groups that will produce inorganic salts. CSH (calcium silicate hydrate) micropore formation in the hardening process of MTA can also facilitate the absorption of ions driven by capillary forces in the matrix. The setting time of the cement paste also increased as the concentration of chitosan in the hydration fluid of the bone cement increased [35]. Maharti et al. (2022) stated that adding water-soluble chitosan into tricalcium silicate-based cement can increase the film thickness, which affects the setting time [36]. In this study, the longest setting time was for MTA-CW10. Wang et al. (2014) also found that adding more than 5% chitosan can slow the setting time of calcium silicate cement. This is due to chitosan’s ability to absorb water excessively and delay the diffusion of water to the anhydrous phase, inhibiting CSH formation. In addition, a high concentration of chitosan can form a thick and dense polymer, enclosing the cement that has not been hydrated yet, inhibiting the interconnection between components so that hydration occurs longer [22]. It can be concluded that at high concentrations, chitosan carries many polyanions, disturbing the balance of cement components and the hydration process, thus slowing the cement’s setting time.
A study showed that adding water-soluble chitosan affects the compression strength of MTA cement. This result agrees with the research of Wang et al. (2014), which showed a significant difference between the treatment and control groups, with a tendency to increase compression strength in the treatment group [22]. However, the tendency to increase compression strength was only found at 2.5% and 5% concentrations. At concentrations 7.5% and 10%, the trend of compression strength decreased as the concentration increased, but it was higher than the control group [22]. Compression strength differs because some concentrations with appropriate COS have dense and compact cement structures. COS particles can fill the empty spaces formed between hydration products. At excessive concentrations, COS can cause porous formation in bone hybrid cement [26, 27]. Although the study of Wang et al. (2014) and this study differed in the variables and methods of chitosan addition, it can be concluded that the concentration of water-soluble chitosan affects the compression strength of calcium silicate-based cement. In this study, the antibiofilm activity of MTA against E. faecalis was assessed in terms of viability, growth, and biofilm formation using the MTT assay.37 The viability value of E. faecalis biofilm in the MTA-CW5 and MTA-CW10 groups was lower than that of MTA. Until now, only a few studies have tested adding water-soluble chitosan to MTA. Nevertheless, this study’s results align with Beshr et al.’s (2019) study. Hiremath et al. (2020) showed that adding acid-soluble chitosan to MTA increased the antibacterial activity against E. faecalis ATCC 29212 [37-39]. The positive charges of chitosan molecules bind to negatively charged components of the bacteria, such as proteins, fatty acids, and phospholipids. These interactions disrupt cell permeability, rupturing organelles and blocking nutrients from entering the bacterial cell, resulting in bacterial cell death; this leads to its antibacterial effect. Chitosan also has a high chelation capacity on various metal ions. Bacterial metalloprotease enzymes bind metal ions as a source of nutrition and metabolism for bacterial cells. Chitosan, a chelating agent, will bind the metal ions so that the bacterial cells are killed through starvation [40]. The increased antimicrobial activity of MTA with chitosan may result from the synergistic forces of biomolecular properties (molecular weight, charge, and degree of deacetylation), material pH (and/or environment), and chitosan-metal complexes. In this study, adding 10% water-soluble chitosan could significantly improve the antibiofilm ability of MTA against E. faecalis. At 5% concentration, chitosan added to MTA increased the antibiofilm activity against E. faecalis, but not significantly. This study’s results align with the research by Abusrewil et al. (2021), showing that adding 2.5% and 5% water-soluble chitosan increased the antibacterial activity of Biodentine on biofilms of three species (S. gordonii, P. gingivalis, and F. nucleatum). The superior effectiveness of this concentration may be attributed to the higher water content of the mixture. The positively charged ions of chitosan could denature the cell wall of the bacteria, and the water molecules hydrolyze the bacteria. Higher water content is found at these concentrations. Moreover, the high chitosan content may be attributed to stronger denaturation power due to increased positively charged ions but with lesser antibacterial power due to lesser water content. While those concentrations with low chitosan concentration may have weaker denaturation power due to lesser positively charged ions but stronger antibacterial power due to more water content for hydrolysis.
Liu et al. evaluated the effects of molecular weight and chitosan concentration on E. coli. At the same molecular weight, chitosan had good antimicrobial activity at high concentrations (200 ppm), and all specimens at low concentrations (20 ppm) could enhance the growth of E. coli. This is due to the bactericidal effect of chitosan at high concentrations occurring through bacterial flocculation. Low concentrations of chitosan did not cause this effect but increased bacterial reproduction [40].
CONCLUSION
Adding various concentrations of water-soluble chitosan to MTA affected the setting time, compression strength, and antibiofilm activity against E. faecalis. The null hypothesis was rejected. The setting time of MTA was faster with 5% water-soluble chitosan, but adding 10% water-soluble chitosan slowed the setting time of MTA. Meanwhile, the compression strength and antibiofilm activity against E. faecalis increased as the concentration of water-soluble chitosan increased.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| BHI | = Brain Heart Infusion |
| MTA | = Mineral Trioxide Aggregate |
ETHICAL STATMENT
This study was approved by the Commission of Ethical Research in Dentistry, Faculty of Dentistry Universitas Indonesia, Number: 05/Ethical Exempted/FKGUI/II/2022 with protocol number: 050240222, Number: 07/05/Ethical Exempted/FKGUI/II/2022 with protocol number: 050220222.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.


