All published articles of this journal are available on ScienceDirect.
Stability and Cytotoxicity Assessments of Hypericum Perforatum Nanoemulsion in Preventing Denture-related Stomatitis
Abstract
Background
There is a growing interest in natural antimicrobials based on herbs, which can be used to prevent and treat oral and dental infections.
Aims
The aim of this study was to evaluate the stability and the cytotoxicity of a Hypericum perforatum nanoemulsion used to prevent denture-related stomatitis.
Methods
The ultrasonic method was used to prepare a Hypericum perforatum nanoemulsion. The prepared nanoemulsion was then characterized using conventional methods. The emulsion resistance to centrifugation was studied, and the formulation was kept at room temperature for 2 months, and any phase separation was checked. Besides, the changes in droplet diameter, polydispersity index (PDI), and surface charge were compared at different time intervals. Finally, the cytotoxicity test was conducted to determine the toxicity of the prepared nanoemulsion against human gingival fibroblasts (HGFs).
Results
The prepared nanoemulsion demonstrated proper physicochemical properties with no cytotoxicity against HGFs. The droplet size, PDI, and surface charge of the nanoemulsion showed a slight increase after 30 days (P=0.6). After 60 days, the increase in the droplet size, PDI, and surface charge were significant (P=0.03). However, the droplet size was still preserved below 200 nm, and the PDI was less than 0.7. Nanoemulsion also showed no physical changes or phase separation after centrifugation and two months of keeping at different temperatures.
Conclusion
The prepared nanoemulsion can be used to prevent and treat oral and dental infectious diseases like denture-related stomatitis.
1. INTRODUCTION
Despite the rapid advances in the technology of polymers, polymethyl methacrylate is still the most common material in prosthetics [1]. Indeed, the main and perhaps economical material to replace teeth is polymethyl methacrylate [2]. Over time, despite the improvement in the mechanical and chemical properties, a large number of incidences regarding microbial and fungal growth have been reported due to dental acryls [3]. Therefore, the antimicrobial and fungal properties of dental acryls have been given great importance [4].
Denture-related stomatitis is among the common infections of acrylate resins, which are in the form of mouth sores with diffuse inflammation (mostly in the maxillary areas) [5]. In more than 70 percent of cases, stomatitis is associated with Candia albicans, and common treatments for Candida albicans have short-term effects and are a definitive treatment for patients [6].
Medicinal treatments for denture-related stomatitis include nystatin, amphotericin B (in more severe cases), fluconazole, and chlorhexidine [6, 7]. However, due to the increasing number of bacterial resistance to antibiotics and the side effects of chemical drugs, the use of medicinal herbs and herbal extracts has increased [8].
There is a growing interest in natural antimicrobial and fungal agents based on medicinal herbs, which can be used to prevent and treat infectious diseases, especially when AMR (Antimicrobial Multi Resistance) threatens human and animal health globally [9]. Natural and environmentally friendly antimicrobial materials are harmless to humans and other organisms, do not cause environmental pollution, and are economically viable [10]. These materials have different mechanisms for fighting the microorganism, including preventing the construction of cell walls and neoclassical acids and inhibiting proteinization and changes in cell membrane function [11]. The clinical effectiveness of plant-based materials in the management of denture stomatitis was suggested by Tatapudi et al. [12].
Hypericum perforatum is herbaceous and perennial and belongs to the Clusiaceae family, which is used in common medicine and herbal therapy due to its antiseptic effects [13]. It has also been found to have anti-bacterial, antiviral, anti-inflammatory, and analgesic activities [14]. Its extract contains flavonoids and phenolic acids, which show the activity of inhibiting free radicals [15]. The efficacy of Hypericum perforatum extract was reported on recurrent aphthous ulcers by Motallebnejad et al. [16]. Moreover, the findings of Antoniadou et al. suggested a promising avenue for Hypericum perforatum in the progress of new antimicrobial plans against oral diseases [17].
Nanomaterials, such as nanoemulsions, provide many benefits in antimicrobial materials, such as reducing antimicrobial use, increasing efficiency, high adaptability to the environment, and improving quality [18]. Research previously conducted to address this problem has included the use of silver nanoparticles for acryl. Acryls containing silver nanoparticles exerted a stronger antifungal effect on standard Candida albicans and had less effect on hospital strains [19].
The aim of this study was to evaluate the stability and cytotoxicity of a Hypericum perforatum nanoemulsion used to prevent denture-related stomatitis.
2. MATERIALS AND METHODS
2.1. Preparation of Hypericum Perforatum Nanoe-mulsion
Tween 80 (2% w/w, Sigma Ultra, low peroxide) was slowly added to Hypericum perforatum oil (4% w/w, Barij Esans Co, Tehran, Iran) and stirred at 1000 rpm. Then, distilled water (Takrou, Tehran, Iran) was added slowly to the mixture, and the mixture was stirred for 10 minutes to form initial droplets. The coarse droplet of the emulsion was then converted to nanometer dimensions by an ultrasonic device (Tomey, Erlangen, Germany, 400 W, 20 KHz) [20].
2.2. Droplet Size and the Polydispersion Index (PDI)
The droplet diameter and PDI of the prepared nanoemulsion were studied using the dynamic light scattering device (DLS, Malvern Instruments, Malvern, UK). For this purpose, a milliliter of the nanoemulsion was diluted with water (5 times) and then poured into the tube of the DLS device. The test was done at different time intervals (immediately after, one month later, and two months after the nanoemulsion was prepared).
2.3. Surface Charge Measurement
For this test, we used zeta potential measurement via a zeta-sizer device (Malvern Instruments, Malvern, UK). One ml of the nanoemulsion was diluted with water (5 times) and then poured into the tube of the zeta-sizer. The test was done at different time intervals (immediately after, one month later, and two months after the nanoemulsion was prepared).
2.4. Morphology Study
The morphology of the prepared nanoemulsion was determined by transmission electron microscopy (TEM, Philips, TecnaiG220). For this purpose, 1 ml nanomaterial (10 times diluted with distilled water) was placed on the carbon plate of the microscope at room temperature. The sample was painted with the help of phospho-tungsten acid (2%), and the image was taken. The test was done at different time intervals (immediately after, one month later, and two months after the nanoemulsion was prepared).
2.5. Stability Assessment
The prepared nanoemulsion was centrifuged for 15 minutes at 4,000 rpm, and the emulsion resistance to centrifugation was tested. In addition, formulations were kept at room temperature for 2 months, and any phase separation was checked (at − 20, 4, and 25°C). Besides, changes in droplet diameter, PDI, and surface charge were compared at different time intervals.
2.6. Assessment of the Cytotoxicity
Human Gingival Fibroblasts (HGFs) were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with fetal bovine serum and antibiotics. Cells were maintained at 37°C in a humidified atmosphere with 5% CO2. HGFs was purchased from Pasteur Institute of Iran (Tehran, Iran). Cells were seeded into 96-well plates at a density of 104 cells per well. After 24 hours of incubation, cells were treated with varying concentrations of the prepared nanoemulsion for 24, 48, and 72 hrs. Untreated cells served as the control. Following the treatment period, the medium was removed, and cells were washed with PBS. Then, 50 microliters MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) solution was added to each well. The plates were then incubated at 37°C for 4 h. After incubation, the MTT solution was carefully aspirated, and the formazan crystals formed by viable cells were solubilized using dimethyl sulfoxide (DMSO). The absorbance was measured at 540 nm using a microplate reader, and the percentage of living cells was evaluated by comparing the control.
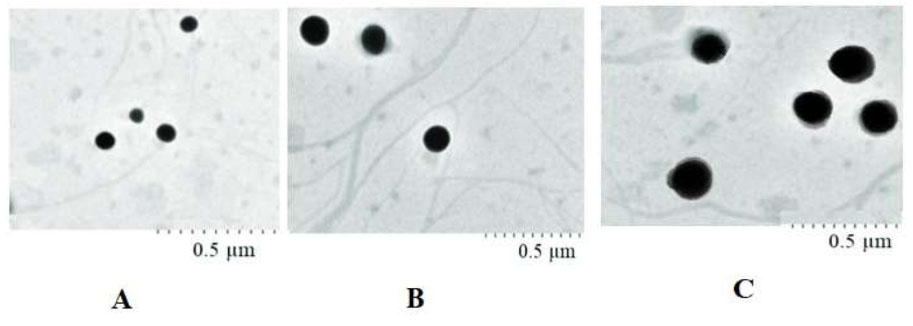
The results for transmission electron microscopic [TEM] images immediately after [A], one month later [B], and two months after [C] the nanoemulsion was prepared.
2.7. Ethical Considerations
The present study entered into the operation phase after the approval of the Ethics Committee of Tabriz University of Medical Sciences with the ethics ID of IR.TBZMED.VCR.REC.1402.018.
2.8. Statistical Analysis
Results were reported as descriptive indicators. To check the normalization of the data, the Shapiro-Wilk test was conducted. In order to compare the groups, the Kruskal-Wallis test was carried out. For data analysis, SPSS software was used. The value of 0.05 was considered to be a significant level.
| Time | The Mean Particle Size [nm] | PDI | The Surface Charge [mV] |
|---|---|---|---|
| Immediately | 92±1.2 | 0.4±0.9 | -33 |
| 30 days | 101±1.5 | 0.5±1.1 | -30 |
| 60 days | 130±1.2 | 0.7±1.4 | -23 |
3. RESULTS
Table 1 presents the results for the mean particle size, PDI, and the surface charge immediately after, one month later, and two months after the nanomaterials were prepared. Fig. (1) shows the results for transmission electron microscopic (TEM) images immediately after, one month later, and two months after the nanoemulsion was prepared. Table 2 presents the results for the cell viability percent after 24, 48, and 72 hours. The prepared nanoemulsion was non-cytotoxic against Human Gingival Fibroblasts (HGFs) at studied times according to ISO 10993-5 [21].
| Time | Control Cell Viability Percent | Nanoemulsion Cell Viability Percent | P-value |
|---|---|---|---|
| 24 h | 100±0 | 98.2±1.2 | 0.6 |
| 48 h | 99.9±0.6 | 97.3±1.3 | 0.7 |
| 72 h | 99.9±0.8 | 97.0±0.9 | 0.7 |
4. DISCUSSION
Droplet size greatly influences the in vivo fate of the nanoemulsion [22]. So, it is essential to control the droplet diameter of the nanoemulsion systems. Small PDI values (PDI < 0.7) specify a moderately narrow size distribution of nanomaterials [23]. Zeta potential values higher than ±30 mV show suspension stability [24]. Wei et al. reported similar results for tea tree oil nanoemulsion [20]. The results of this study showed spherical monodispersed droplets for the prepared nanoemulsion. The droplet size showed a slight increase after one month. After 60 days, the increase in the droplet size was significant (P<0.05).
The creaming, sedimentation, and phase separation of emulsion systems can be evaluated by a centrifugation test to ensure their stability [25]. As mentioned in Table 1, the droplet size, PDI, and surface charge of the nanoemulsion at two steps showed a slight increase (P=0.6). After 60 days, the increase in the droplet size, PDI, and surface charge were significant (P=0.03). However, the droplet size was still maintained below 200 nm, and the PDI was less than 0.7. Nanoemulsion also presented no physical alterations or phase separation after 4000 rpm centrifugation force. Moreover, it showed good stability when exposed to three different temperatures (− 20, 4, and 25°C) for 60 days. In a study by Wei et al., tea tree oil nanoemulsion showed no physical changes or phase separation after centrifugation [20].
The prepared nanoemulsion was non-cytotoxic against Human Gingival Fibroblasts (HGFs) at studied times according to ISO 10993-5 [21]. Khadem Nezhad et al. examined the cytotoxicity influence of Hypericum perforatum oil against human gingival fibroblasts. The results showed that the concentration of 0.625μg/ml of Hypericum perforatum did not have toxic effects on gingival fibroblast cells. Therefore, it can be used as a natural mouthwash product [26].
New findings in the dental field can advance the quality of life even in particularly complex patients [27]. For example, mucoadhesive polymeric oral drug delivery carriers can be suitable pharmaceutical agents [28, 29]. They can also functionalized to improve cell-specific interactions in the small intestine to enhance retention time and uptake [30]. Colombo et al. [30] and Aljuanid et al. [31] investigated mucoadhesive nanoemulsion patches for oral drug delivery.
CONCLUSION
In this study, the prepared Hypericum perforatum nanoemulsion exhibited excellent physicochemical properties, good stability, and no cytotoxicity against HGFs. The prepared nanoemulsion can be used to prevent and treat oral and dental infectious diseases like denture-related stomatitis in the near future. As a main future outlook, the utilization of a mucoadhesive carrier with sustained release antimicrobial and antifungal effects could significantly improve clinical outcomes.
AUTHORS' CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to itssubmission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| PDI | = Polydispersity index |
| HGFs | = Human gingival fibroblasts |
| DLS | = Dynamic light scattering device |
| DMEM | = Dulbecco's Modified Eagle Medium |
| DMSO | = Dimethyl sulfoxide |
| TEM | = Transmission electron microscopy |
ETHICAL STATEMENT
The present study entered into the operation phase after the approval of the Ethics Committee of Tabriz University of Medical Sciences with the ethics ID of IR.TBZMED.VCR.REC.1402.018.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this research are available within the article.
FUNDING
This study extracted the data from a proposal recorded at the Tabriz University of Medical Sciences (number 70125).


