All published articles of this journal are available on ScienceDirect.
Assessment of Sticky Bone in One- Stage Lateral Sinus Lift Procedures: A 4 year Retrospective Study
Abstract
Purpose
This study aimed to assess the clinical and radiographic outcomes of using sticky bone in lateral sinus lift procedures combined with dental implant placement, and compare the clinical and radiographic outcomes between the perforated and non-perforated groups.
Methods
This retrospective study was conducted on individuals who presented to the Department of Oral and Maxillofacial Surgery at Tishreen University from September 2018 to September 2022. The sample included patients who had lateral sinus lift with simultaneous dental implant placement. The lateral sinus floor elevation procedures involved the use of sticky bone, a mixture of injectable platelet-rich fibrin, and bovine graft material. Bone height measurements were assessed using preoperative and 9-month postoperative CBCT scans. Statistical analyses were conducted to evaluate the Initial Bone Height (IBH), bone height after 9 months, and Intra-sinus Bone Gain (IBG). The intra-sinus bone gain outcomes were compared between perforated and non-perforated groups using an unpaired t-test.
Results
In this study, 21 implants were inserted in 15 patients who underwent lateral sinus augmentation using sticky bone. Four membrane perforations were identified during 15 procedures (26.6%). The survival rate for all implants was 100%. The average intra-sinus bone gain was 5.54 ± 0.74 mm. There was no statistically significant difference in intra-sinus bone gain between the perforation group and non-perforation group (P>0.001).
Conclusion
Based on the findings of this study, sticky bone can be effectively used as a graft material to achieve bone graft survival and successful sinus augmentation in lateral sinus lift procedures with simultaneous dental implant placement, even following small and medium Schneiderian membrane perforation (<10 mm).
1. INTRODUCTION
Dental implant placement in the posterior maxilla poses challenges for practitioners due to characteristics of this region, including deficient available bone and low bone density [1]. Various approaches are available to rehabilitate posterior maxilla for dental implant placement. These approaches can be categorized into grafting options, such as veneer and onlay grafts, guided bone regeneration techniques, and sinus lifting, as well as non-grafting options, such as short, titled, or zygomatic implants [2].
Maxillary sinus lifting is a common and reliable surgical approach used to rehabilitate the posterior maxilla [3]. Implant survival rates in areas where maxillary sinus augmentation is performed are similar to those achieved in pristine bone [4]. Maxillary sinus lifting can be performed using various grafts, including autogenous, allograft, xenograft, alloplastic bone, and platelet concentrates [5, 6].
Schneiderian membrane perforation is the most common complication of lateral sinus lifting, with a reported rate ranging from 10% to 60% [7]. The integrity of the sinus membrane is a critical factor in preventing bacteria invasion and preserving graft material [7]. Membrane perforation can occur due to factors such as uncontrolled dissection, reduced membrane thickness, decreased friability and elasticity, inappropriate instruments, presence of the sinus septa, and lack of experience [8].
Repairing a perforated membrane poses a challenge for practitioners. Authors have reported several repair techniques, including placing an absorbable membrane over the perforation, using resorbable hemostatic agents, employing platelet-rich fibrin, utilizing fibrin glue, folding the membrane itself, and suturing the membrane with resorbable material [7].
Sticky bone, also known as a mineralized plasmatic matrix, is an autologous fibrin network that incorporates a bone substitute [9, 10]. This gelatinous structure is easy to malleable, offering stability and volume preservation. It also contains leukocytes and platelets that release growth factors [11]. It can be prepared by combining bone substitutes with platelet concentrates that are initially in a liquid state, transforming into a gelatinous mass. These platelet concentrates include Platelet-Rich Plasma (PRP), Plasma-Rich in Growth Factors (PRGF), and injectable platelet-Rich Fibrin (iPRF) [10, 11]. Given these characteristics, we hypothesize the potential utility of sticky bone in managing Schneiderian membrane perforations (<10 mm) .This retrospective study aimed to assess the effectiveness of using sticky bone in lateral sinus lift augmentation procedures.
2. MATERIALS AND METHODS
2.1. Patients' Selection and Presurgical Evaluation
This retrospective study adhered to the principles of the Declaration of Helsinki for human research and received approval from the Ethics Committee of Tishreen University with number ref 323 on 15-3-2023. This work is fully compliant with the STROBE criteria. Patients were provided with comprehensive information regarding the surgical procedures, and all participants provided written informed consent before being included in the study. This research is registered with the Research Registry under the identification number researchregistry9008.
This study was conducted on patients who had reported to the department of Oral and Maxillofacial Surgery at Tishreen University between September 2018 and June 2022. Patient files were carefully reviewed to identify individuals who met the following inclusion criteria.
2.1.1. Inclusion Criteria
1- missing multiple maxillary posterior teeth. 2- residual bone height (3-5) mm, 3- CBCT scans available before and 9 months after sinus augmentation, 4- Lateral sinus lifting using a sticky bone as a grafting material with simultaneously dental implant placement, 5- Schneiderian membrane perforation size<10 mm.
2.2. Surgical Procedure
2.2.1. Preparation of Sticky Bone
A 20 mL of venous blood was taken from the patient's forearm and collected into two non-coated plastic tubes (without silica) (BD Vacutainer®, Becton Dickinson, United Kingdom). The tubes were then immediately placed in a centrifuge (Intralock International, Birmingham, USA) at 700 rpm for 3 min. After the completion of centri- fugation, two distinct layers were observed in the tube: the upper layer containing yellow plasma liquid (Injectable platelet-rich fibrin) and the red blood cells settling at the bottom of the tube. Injectable Platelet-Rich Fibrin (iPRF) was mixed with xenograft (Bio-Gen; Bioteck Co, Germany) on a glass dish (Fig. 1). The formation of sticky bone occurs through polymerization, a process that usually takes around 10-15 minutes.

Preparation of sticky bone by mixing iPRF with bovine graft.
2.2.2. Lateral Sinus Lift Procedure
All surgical interventions were carried out by the same surgeon under local anesthesia, which included 2% lidocaine and 1:100,000 epinephrine (Lidocain, AVENZOR, Syria). A crestal incision and two incisions were made. A full-thickness flap was raised to reveal the alveolar ridge. The lateral window was created using a round diamond bur, followed by careful dissection and elevation of the Schneiderian membrane using manual instruments (Fig. 2A). Visual inspection and the Valsalva maneuver were employed to assess the integrity of the sinus membrane. (Fig. 2B). Membrane perforation was managed by folding the membrane itself. The implant sites was prepared according to the manufacturer's instructions (INNO submerged implant; Cowellmedi Inc, South Korea). The implants were placed using a motorized handpiece, ensuring that all implants were positioned at the level of the crestal bone (upper surface of implant's shoulder at the level of crestal bone) (Fig. 2C). The previously prepared sticky bone was used to fill the sub-antral space (Fig. 2D). Following implant placement, the flap was repositioned, and sutures were used to close the incisions with 4-0 silk suture (SilkoMed; MedSilk GmbH, Germany). Patients were prescribed amoxicillin/clavulanate 875/125 mg (Augmentin 1000, Maatouk Pharma, Syria) twice daily for 5 days and potassium diclofenac 50 mg (Flam K, Asia Co, Syria) as required. Suture removal was performed one week postoperatively (Flowchart – Supplementary).
2.3. Radiographic Analysis
The CBCT scan (Carestream Dental CS 9600 LLC, Atlanta, GA, USA) was used to evaluate the specified variables both before the operation and 9 months after the surgery (Fig. 3).
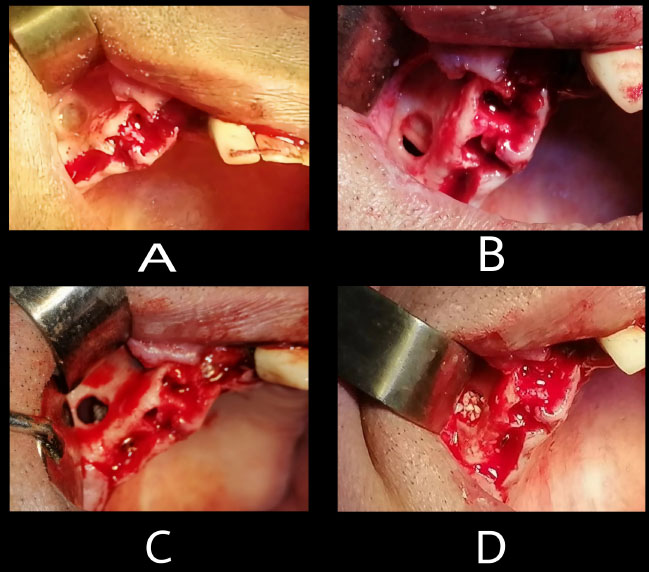
Lateral sinus lift procedure: (A) Lateral window creation, (B) sinus membrane perforation was occurred during membrane dissection, (C) Implant placed in prepared osteotomy sites, (D) residual sinus space filled with sticky bone.
Initial bone Height (IBH) was determined at postoperative CBCT by measuring the distance between the alveolar bone crest and the sinus floor at the intended implant placement sites.
Bone height after 9 months was determined at postoperative CBCT by measuring the distance between the implant's shoulder and the new sinus floor at the same site.
Intra-sinus bone gain refers to the difference between the bone height after 9 months and the Initial Bone Height measured initially.
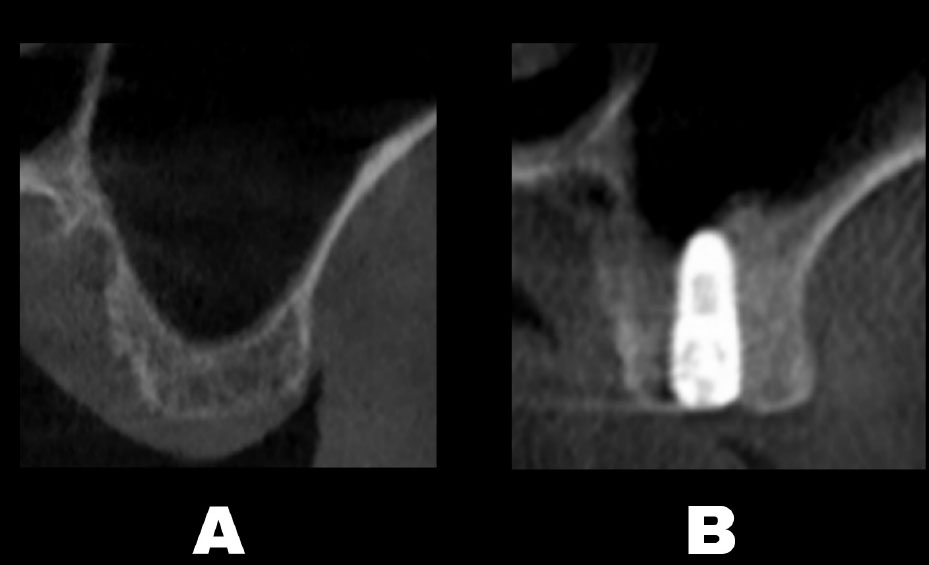
CBCT was performed before surgery (A), and after 9 months (B).
2.4. Statistical Analysis
The statistical analysis was performed using SPSS version 22 software (SPSS Inc., IL, USA). Descriptive statistics were utilized, including the mean and standard deviations, to evaluate the Initial Bone Height (IBH), bone height after 9 months, Intra-sinus Bone Gan (IBG). The differences in bone height between the two time periods were evaluated using a paired t-test. Intra-sinus bone gain outcomes were compared with an unpaired t-test. The level of significance considered was 5% (α=0.05).
3. RESULTS
3.1. Patient Characteristics
This retrospective study involved 15 patients (9 males, 6 females) with an average age of 45.3 years (range, 37–57 years) who met the inclusion criteria for the study.
3.2. Clinical Analysis
The survival rate for all implants was 100%. Four membrane perforations were observed in 4 out of 15 procedures (26.6%). There were no other intraoperative complications noted, and no postoperative complications observed.
3.3. Radiographic Analysis
The initial bone height varied from 3.3 to 4.8 mm, with an average of 4.13 ± 0.44 mm. The bone height after 9 months ranged from 8.2 to 11.3 mm, averaging 9.68 ± 0.81 mm. Intra-sinus bone gain ranged from 4.6 to 7.1 mm, with a mean of 5.54 ± 0.74 mm (Table 1).
The difference between the Initial Bone Height and the bone height after 9 months exhibited a statistically significant variance (P < 0.001) (Table 2). There was no statistically significant distinction in intra-sinus bone gain between the perforation group and the non-perforation group (P>0.001) (Table 3).
| - | Initial Bone Height | Bone Height after 9 Months | Bone Gain |
|---|---|---|---|
| Mean | 4.13 | 9.68 | 5.54 |
| SD | 0.44 | 0.81 | 0.74 |
| Max | 4.8 | 11.3 | 7.1 |
| Min | 3.3 | 8.2 | 4.6 |
| Initial Bone Height | Bone Height after 9 Months | Paired T-Test | P-Value |
|---|---|---|---|
| 4.13 | 9.68 | 28.78 | < 0.001 |
| Variable | Non-perforation Group | Perforation Group | p-Value |
|---|---|---|---|
|
intra-sinus bone gain |
5.58 | 5.45 (±) | 0.775 (< 0.001) No statistically significant difference |
| 0.752 | 0.835 | ||
| Range: (4.6 ; 7.1) | Range: (4.6 ; 6.6) | ||
| N = 11 | N = 4 |
4. DISCUSSION
This four-year retrospective study was conducted with the primary objective of assessing the effectiveness of using sticky bone in external sinus lift procedures in conjunction with dental implant placement. Furthermore, the study aimed to investigate a new approach for treating sinus membrane perforations smaller than 1 cm by utilizing sticky bone without supplementary interventions. The utilization of sticky bone yielded favorable outcomes, characterized by substantial bone augmentation and the absence of noteworthy complications. The study did not identify any statistically significant variances between the perforation group and the group with an intact sinus membrane.
Lateral sinus augmentation has evolved into a commonly employed and dependable procedure for increasing bone height to support dental implant placement in the posterior maxilla. However, this procedure is regarded as intricate and requires surgical skill, particularly during Schneiderian membrane dissection. Research suggests that Schneiderian mem- brane perforation is the most prevalent complication, with reported rates ranging from 7 to 60% [12, 13]. Studies have highlighted the importance of preventing these perforations from ensuring favorable outcomes. This can be accomplished by conducting a comprehensive assessment of factors that may elevate the risk of this complication [14, 15].
Maintaining the integrity of the Schneiderian membrane and ensuring effective closure of any perforations are key factors for successful bone augmentation procedures in the maxillary sinus [16]. This is due to the critical function of the membrane as a biological barrier, which serves to prevent bacterial invasion, infection, and graft loss in the maxillary sinus [17]. In addition to the barrier function, some experimental studies have suggested that the sinus membrane may also play a regenerative role by serving as a source of osteoprogenitor cells [18]. However, several histological studies have disputed this regenerative capacity and have suggested that it is relatively insignificant [19, 20], which is consistent with our results, as we observed no significant differences in bone gain between the perforation group and the non-perforation group.
The impact of Schneiderian membrane perforation on clinical outcomes and postoperative complications remains a subject of debate due to conflicting results from different studies. Several studies [9, 21, 22] have reported no statistically significant differences in postoperative complications and implant success rate in augmented sinus areas between patients with perforated sinus membranes and those with intact membranes, aligning with our findings. However, other studies have reported statistically significant differences in clinical outcomes [23, 24].
Throughout recent decades, numerous techniques have been suggested to address Schneiderian membrane perforations, each with its own indications and considerations. The treatment technique is determined by the location and size of the perforation [25]. Membrane suturing is a meticulous technique that requires careful detachment of the Schneiderian membrane from the bony walls to achieve a tension-free closure [25].
An alternative method for managing small perforations involves carefully lifting up more of the undamaged part of the membrane so that it folds over itself [7, 26]. The authors suggested that the specific intervention is generally unnecessary, as the simple reflection of the membrane can obliterate the perforation [26, 27]. This method is often combined with the utilization of resorbable barrier membranes [7]. Although barrier membranes offer advantages, their utilization comes with various limitations, such as high expenses, challenges in stabilization, uncertain resorption rates, and the possibility of containing chemical residues that may trigger unwanted inflammatory reactions [28].
It is recommended that the surgical procedure be suspended in cases of large membrane perforations or complete membrane opening. In such situations, a reentry procedure may be considered, but only after a healing period of no less than 6-8 weeks [29].
Platelet Rich Fibrin (PRF) membranes have been utilized in managing Schneiderian membrane perforation due to their autologous properties as a bioactive substance [30, 31]. Platelets within PRF gradually release various proteins and growth factors that can aid in bone healing and also regulate inflammation processes [32, 33]. Aricioglu [34] reported no significant differences in Schneiderian membrane healing when using PRF or collagen membranes.
Sticky bone refers to an autologous blood product containing a high concentration of platelets and fibrin that is mixed with bone graft material to form a homogenous gelatinous component [11]. Sticky bone is simple to handle and can be applied to any bone defect without scattering bone graft material. Its use enables the stabilization of the bone graft within the defect, reducing micromovement and preventing bone loss during the healing process [35]. The platelets and leukocytes trapped within the fibrin network serve as sources of growth factors and various proteins. Therefore, utilizing sticky bone derived from xenografts or allografts can provide an advantage by acquiring the osteoinductive properties typically found in autogenous bone [35-40].
This paper described the first study evaluating the use of sticky bone in managing Schneiderian membrane perforations during lateral sinus lift procedures. The study's limitations include 1- the absence of histological study of the newly formed bone, and 2- the lack of follow-up period post-functional loading.
CONCLUSION
Based on the findings of this study, sticky bone can be effectively used as a graft material to achieve bone graft survival and successful sinus augmentation in lateral sinus lift procedures with simultaneous dental implant placement, even following small and medium Schneiderian membrane perforation (<10 mm).
AUTHORS' CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to itssubmission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| iPRF | = Injectable platelet rich fibrin |
| CBCT | = Cone-Beam Computed Tomography |
| IBH | = Initial bone height |
| IBG | = Intra-sinus bone gain |
| PRF | = Platelet rich fibrin |
| PRP | = Platelet-rich plasma |
| PRGF | = Plasma-rich in growth factors |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This retrospective study was approved by the Ethics Committee of Tishreen University, Syria, with number ref. 323 on 15-3-2023.
HUMAN AND ANIMAL RIGHTS
This retrospective study was conducted in accordance with the Helsinki Declaration, which was revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the [Zenodo] at [zenodo.org], reference number [Doi: 10.5281/zenodo.11476578].


