All published articles of this journal are available on ScienceDirect.
Prevalence of Dentin Hypersensitivity Among Dental Students and Effectiveness of Tooth Desensitizing Agents
Abstract
Objectives
Dentin hypersensitivity (DH) is a growing concern in Dentistry. The objective of this study was to evaluate the prevalence and effectiveness of different desensitizing agents of (DH) among undergraduate dental students.
Materials and Methods
This cross-sectional clinical study was conducted with 161 undergraduate dental students. A self-reporting questionnaire along with a clinical examination was performed to diagnose DH and determine the severity. The effectiveness of home-based and in-office desensitizers was evaluated by comparing the combined DH scale in the pre- and post-treatment periods. The data were analyzed for frequency, correlation, and T-test.
Results
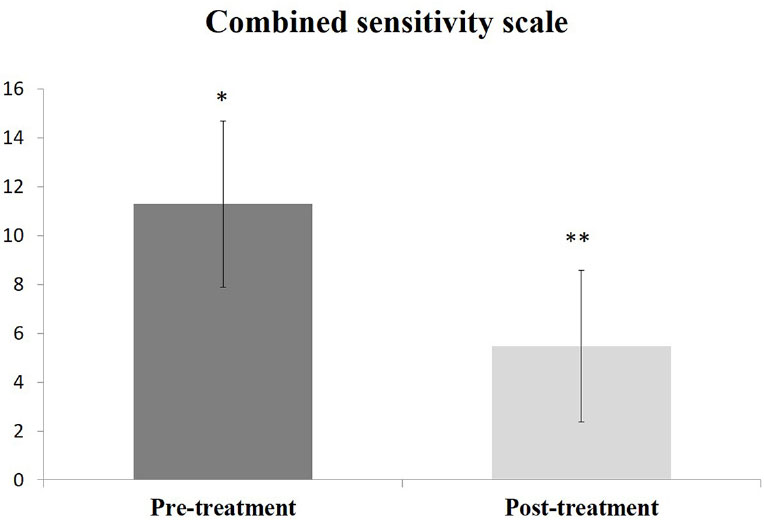
The prevalence of DH in the tested population was 19.3%, predominantly in females. The Chi-Square test showed significance in gender and oral hygiene practice by the participants (p<0.05). However, none of the tested factors strongly correlated with DH in this population. The post-treatment combined DH scale (5.48±3.1) was statistically significant (p=0.0001) compared to the pretreatment scale (11.29±3.5). The effectiveness of SRA was 87%, and the remaining 13% of DH recovered using GLUMA.
Conclusion
The prevalence of DH was 19.3% without any strongly correlated etiology. Home-based and in-office desensitizers were effective in reducing DH in the tested population.
1. INTRODUCTION
Dentin hypersensitivity (DH), often referred to as tooth sensitivity, is a common dental condition characterized by sharp, sudden pain or discomfort in response to various stimuli. This discomfort typically originates from the exposed dentin, which is the inner layer of the tooth, and can be triggered by activities such as eating, drinking, brushing, or even breathing cold air. [1] While DH is a widespread condition and considered quite bothersome, the proposed treatments for it have proven to be inadequate and not highly effective [2]. The prevalence of dentin hypersensitivity can vary depending on the population studied, geographic location, and other factors [3]. The condition is common among patients aged 20-50, but it is notably more prevalent within the 30-40 age group and particularly among females. This higher prevalence among females may be associated with their dental care and dietary habits [4, 5]. Additionally, dentin hypersensitivity is more prevalent in canine and premolar teeth compared to other teeth [6, 7]. It has been documented that the disease predominantly affects the buccal surface of the teeth compared to other areas [8]. There are two widely used approaches for assessing the severity of DH. One involves administering questionnaires to patients, while the other entails a clinical examination. Typically, the prevalence distribution of DH is more frequently relied upon when using the first method, as it is simpler and more time-efficient compared to the second method [9].
Dentin is recognized as a living tissue capable of reacting to both natural and abnormal stimuli. Loss of enamel and gingival recession caused by tooth attrition and fracture can result in dentin exposure, which can lead to the development of DH [10]. Exposed dentinal tubules are the main source of pain. Dentin sensitivity is thought to be caused by three major mechanisms [11]. Three theories are relevant here: The Direct Innervation Theory, the Odontoblast Receptor Theory, and the Fluid Movement/Hydrodynamic Theory. According to the Direct Innervation Theory, nerve terminals penetrate the dentin through the pulp and extend to the DEJ, where mechanical stimuli immediately transmit pain. However, there is limited evidence supporting this theory. The second theory is the Odontoblast Receptor Theory, suggesting that odontoblasts function as pain receptors that transmit signals to pulpal nerves. Nevertheless, this theory has been refuted due to the inability of the cellular matrix of odontoblasts to generate neuronal impulses. Barnstorm originally proposed the Hydrodynamic Theory for sensitive dentin [12]. The most frequently held DH theory is this one. The theory has been founded on the concept that the flow of fluid inside the dentinal tubules serves as its foundation. According to this idea, the tubules remain open from the visible dentin surface to the pulp [13].
In order to reduce dentin hypersensitivity, a range of recommended desensitizing treatments are available, which encompass nerve-desensitizing substances, protein-precipitating agents, dentin adhesive sealants, agents that block dentinal tubules, as well as homeopathic remedies [14]. DH is currently treated by blocking dentinal tubules, isolating external stimuli, preventing tubular fluid flow, and lowering pulp nerve fiber responsiveness. Fluoride, arginine, calcium-containing compounds, and strontium chloride compounds are among the active components in desensitized toothpaste [15]. Dentinal hypersensitivity can be treated with a variety of in-office treatments or by the patient applying desensitizing medications at home [16]. However, there is a wide range of therapeutic options and desensitizing drugs available, and there is inadequate evidence to compare their relative effectiveness. It is crucial to research the efficacy of desensitizing agents in order to figure out how to lessen the severity of DH [17].
The objectives of the present study were to evaluate the prevalence of tooth hypersensitivity among undergraduate dental students, evaluate the factors leading to tooth hypersensitivity, and the effect of different desensitizing agents on this population.
2. MATERIALS AND METHODS
2.1. Study Design
This research was approved by the Ministry of Health and Prevention UAE RAK-REC approval number MOHAP/REC/2021/ 58-2021-UG-D and the ethical committee of RAKMHSU-REC-025-2021/22-UG-D. This cross-sectional study was conducted at the RAK College of Dental Sciences clinic over a period of 6 months. The study population was undergraduate students studying at RAK College of Dental Sciences in the 2021-22 academic year. The sample size =160 was calculated using an online calculator (Raosoft, Inc.) at a margin of error of 5% and confidence level of 95%. The participants were randomly selected following the inclusion criteria: 1-5-year students of RAK College of Dental Sciences willing to participate in the study voluntarily. The exclusion criteria were participants with systemic diseases and participants with known pulp pathology.
2.2. Data Collection
Data was collected using questionnaires provided both in English and Arabic. The questions used in this study were closed-ended questions in the form of MCQ. The procedure was explained to the participants, and written consent was obtained prior to examining the participants. A brief history of oral hygiene practice, food habits, and family history of DH was obtained by the questionnaire. To determine DH among the participants who answered yes or did not know about having DH, a standard dental examination was performed by 2 trained operators. The DH was diagnosed using an evaporative air stimulus, “air blast hypersensitivity,” and tactile and cold stimuli. [18] In a patient with known DH, the severity of the disease was analyzed using a visual analog scale, numeric scale, face pain scale, and verbal evaluation scale [19].
2.3. Disease Intervention
Participants diagnosed with DH were given the first line of treatment with home desensitizing agent Sensodyne rapid action toothpaste (GSK plc. Brentford, United Kingdom). The participants were instructed to use the toothpaste following the manufacturer's instructions. A follow-up reevaluation was conducted after 2 weeks. Participants who did not recover with home desensitizer were treated with in-office desensitizer (GLUMA Kulzer GmbH, Hanau, Germany) and re-evaluated after 2 weeks.
2.4. Data Analysis
The data was analyzed using the statistical software SPSS 24.0 (SPSS IBM, Armonk, NY, USA). The prevalence of DH was calculated using percentages. The demographic data were analyzed using frequency and Chi-square tests, and the associated factors were analyzed using frequency. The correlation of the factor with DH was analyzed using the Spearman Correlation test.
3. RESULTS
A total of 161 students participated in this study. Among them, 63 were male and 98 were female participants. More than 94% of the participants use either soft or medium soft type toothbrushes. Among the participants, 85% of them spent 1-2 minutes brushing their teeth each time. The majority of the participants (70.8%) had a habit of brushing their teeth twice per day, and 52.2% of participants had changed their toothbrushes every 3 months. Among the participants, more than 84% occasionally use additional oral hygiene aids like dental floss/ mouthwash or both. Although the participants used no predominant toothpaste band, Colgate was the most frequently used (31.7%) toothpaste brand among the participants. The demographic data of the participants and their oral hygiene practices are shown in Table 1. Out of 161 participants, 31 participants were diagnosed with DH, making the prevalence 19.3% in the tested population. The prevalence was higher among females, 77.4%, than males, 22.6%. The nature of DH and its severity using different scales are shown in Table 2. Chi-square analysis showed a statistically significant effect of gender (p=0.002), type of toothbrush used by the participant (p=0.002), duration of tooth bushing each time (p=0.006), tooth brushing frequency per day (p=0.001), and the frequency of toothbrush change (p=0.001) on DH. However, when compared with the combined DH scale, none of the factors showed a strong correlation. The factors associated with DH and its significance and correlation of those factors with the combined DH scale are shown in Table 3.
The use of home desensitizer for 2 weeks showed a statistically significant reduction of the combined DH scale (p=0.0001); however, 4 out of 31 patients diagnosed with DH did not recover from home desensitizer. All these 4 cases were responded to and recovered using in-office desensitizer (GLUMA). The combined DH scale before and after 2 weeks of intervention is shown in Fig. (1).
| Variable | Category | N | Percentage |
|---|---|---|---|
| Gender | Male | 63 | 39.1% |
| Female | 98 | 60.9% | |
| Type of tooth brush uses | Extra soft | 6 | 3.7% |
| Soft | 77 | 47.8% | |
| Medium soft | 75 | 46.6% | |
| Hard | 3 | 1.9% | |
| Duration of tooth brushing each time | Less than 1 min | 7 | 4.3% |
| 1 min | 64 | 39.8% | |
| 2 min | 73 | 45.3% | |
| 3 min | 14 | 8.7% | |
| More than 3 min | 3 | 1.9% | |
| Tooth brushing frequency per day | 1 time | 16 | 9.9% |
| 2 times | 114 | 70.8% | |
| 3 times | 29 | 18.0% | |
| 4 times | 2 | 1.2% | |
| Frequency of brush change | 1 month | 14 | 8.7% |
| 2 months | 33 | 20.5% | |
| 3 months | 84 | 52.2% | |
| 6 months | 26 | 16.1% | |
| Others | 4 | 2.5% | |
| Additional oral hygiene aid uses | Floss | 31 | 19.3% |
| Mouthwash | 52 | 32.3% | |
| None | 42 | 26.1% | |
| Floss + MW | 36 | 22.4% | |
| Brand of toothpaste use | Colgate | 51 | 31.7% |
| Signal | 23 | 14.3% | |
| Synsodyne | 40 | 24.8% | |
| Closeup | 12 | 7.5% | |
| Paradontax | 4 | 2.5% | |
| Mix | 31 | 19.3% | |
| Soft drink frequency | 1/day | 37 | 23% |
| 2/day | 20 | 12.4% | |
| 3/day | 9 | 5.6% | |
| 4/day | 3 | 1.9% | |
| 5/day | 2 | 1.2% | |
| 1/week | 23 | 14.3% | |
| 2/week | 26 | 16.1% | |
| 3/week | 26 | 16.1% | |
| 4/week | 7 | 4.3% | |
| 5/week | 8 | 5.0% | |
| Cavity | Yes | 38 | 23.6% |
| No | 95 | 59.0% | |
| Not sure | 28 | 17.4% | |
| Ortho TX | Yes | 58 | 36.0% |
| No | 103 | 64.0% | |
| Family history of hypersensitivity | Yes | 38 | 23.6% |
| No | 89 | 55.3% | |
| Not sure | 34 | 21.1% | |
| Hypersensitivity | Yes | 31 | 19.3% |
| No | 115 | 71.4% | |
| Not sure | 15 | 9.3% |
| Variable | Category | N | Percentage |
|---|---|---|---|
| Visual analogue scale | No pain | 1 | 3.2% |
| 25% pain | 17 | 54.8% | |
| 50% pain | 9 | 29.0% | |
| 75% pain | 4 | 12.9% | |
| Numeric scale | 1 | 2 | 6.5% |
| 2 | 4 | 12.9% | |
| 3 | 9 | 29.0% | |
| 4 | 4 | 12.9% | |
| 5 | 5 | 16.1% | |
| 6 | 5 | 16.1% | |
| 7 | 1 | 3.2% | |
| 8 | 1 | 3.2% | |
| Face scale | Big smile | 2 | 6.5% |
| Smile | 9 | 29.0% | |
| Straight | 11 | 35.5% | |
| Sad | 8 | 25.8% | |
| Very sad | 1 | 3.2% | |
| Verbal evaluation scale | None | 1 | 3.2% |
| Mild | 22 | 71.0% | |
| Moderate | 7 | 22.6% | |
| severe | 1 | 3.2% | |
| Trigger factors | Cold | 19 | 61.3% |
| Hot | 1 | 3.2% | |
| Air | 3 | 9.7% | |
| Multiple | 8 | 25.8% | |
| Duration | Few seconds | 29 | 93.5% |
| Few minutes | 2 | 6.5% | |
| Using hypersensitivity toothpaste | Yes | 8 | 25.8% |
| No | 22 | 71.0% | |
| Not sure | 1 | 3.2% | |
| Name of toothpaste | Sensodyne | 7 | 22.6% |
| Parodontax | 2 | 6.5% | |
| Not applicable | 22 | 71.0% |
| Variable | Category | N | % | Chi-Squire (p=) | Correlation with Combined DH Scale (R=) |
|---|---|---|---|---|---|
| Gender | Male | 7 | 22.6% | 0.002 | -0.022 |
| Female | 24 | 77.4% | |||
| Type of tooth brush uses | Extra soft | 2 | 6.5% | 0.002 | 0.168 |
| Soft | 18 | 58.1% | |||
| Medium soft | 11 | 35.5% | |||
| Duration of bushing each time | 1 min | 11 | 35.5% | 0.006 | 0.053 |
| 2 min | 14 | 45.2% | |||
| 3 min | 4 | 12.9% | |||
| More than 3 min | 2 | 6.5% | |||
| Tooth brushing frequency per day | 1 time | 3 | 9.7% | 0.001 | 0.091 |
| 2 times | 22 | 71.0% | |||
| 3 times | 6 | 19.4% | |||
| Frequency of brush change | 1 month | 2 | 6.5% | 0.001 | -0.123 |
| 2 months | 7 | 22.6% | |||
| 3 months | 16 | 51.6% | |||
| 6 months | 4 | 12.9% | |||
| Others | 2 | 6.5% | |||
| Additional oral hygiene aid uses | Floss | 6 | 19.4% | 0.540 | 0.260 |
| Mouthwash | 8 | 25.8% | |||
| None | 6 | 19.4% | |||
| Floss + MW | 11 | 35.5% | |||
| Brand of toothpaste uses | Colgate | 9 | 29.0% | 0.141 | -0.007 |
| Signal | 5 | 16.1% | |||
| Synsodyne | 10 | 32.3% | |||
| Closeup | 2 | 6.5% | |||
| Mix | 5 | 16.1% | |||
| Soft drink frequency | 1/day | 7 | 22.6% | 0.115 | 0.047 |
| 2/day | 5 | 16.1% | |||
| 5/day | 1 | 3.2% | |||
| 1/week | 4 | 12.9% | |||
| 2/week | 6 | 19.4% | |||
| 3/week | 6 | 19.4% | |||
| 4/week | 1 | 3.2% | |||
| 5/week | 1 | 3.2% | |||
| Presence of cavity | Yes | 11 | 35.5% | 0.369 | 0.167 |
| No | 14 | 45.2% | |||
| Ortho TX | Yes | 13 | 35.5% | 0.369 | -0.004 |
| No | 18 | 45.2% | |||
| Family history of hypersensitivity | Yes | 14 | 45.2% | 0.303 | 0.077 |
| No | 7 | 22.6% | |||
| Not sure | 10 | 32.3% |
4. DISCUSSION
Different studies have identified DH as a highly frequent illness affecting a wide variety of people. It has a high prevalence rate among young adults. There are plenty of studies on the prevalence of DH that are conducted in different parts of the world and on different populations. The results of these studies showed a wide range of prevalence, from 4.8% to 62.3%. A systemic review and meta-analysis conducted by Favaro Zeola et al., 2019, reported the possibility of a high risk of bias in DH prevalence studies [20]. In our study, the participants were within a short-range age group and had similar educational backgrounds, following specific inclusion and exclusion criteria. The standardized clinical examination, including multiple DH tests along with a self-reporting questionnaire, was used to limit the possible bias in determining the prevalence of DH among the target population [21]. The prevalence of DH among dental students was 19.3%. The prevalence was low among the tested population compared to the average DH prevalence reported in the previous studies. Due to a lack of studies conducted on dental students, the current data could not be accurate. However, this could be due to the fact that the studied population is well-versed in hypersensitivity and oral hygiene compared to the general population with varying educational and socioeconomic status [22].
There are plenty of articles published to date on DH. The researcher found several etiological factors to be associated with DH. However, in our study, we could not find any factor strongly correlated with DH among the tested population. Among the tested parameters, the use of additional oral hygiene in the form of dental floss or mouthwash showed a very mild correlation with DH. Oral hygiene practice plays an important role in overall oral health. In order to diagnose DH, it is essential to exclude dental conditions with similar pain symptoms [23]. In our study, majority of the participants were found to have good oral hygiene practices though they were diagnosed as having DH. Although several previous studies have emphasized the association of oral hygiene practice with DH [24]. In our study, we did not find any strong correlation. Previous studies explained that acidic drink consumption causes exposure of dentinal tubules and eventually leads to DH [25, 26]. However, in our study, we could not find any significant correlation between soft drink consumption and DH.
The treatment intervention provided to the participants of our study was a home-based desensitizing toothpaste called Sensodyne Rapid Action (SRA), which showed a high success rate (87%) in reducing DH. SRA contains potassium nitrate, strontium acetate, fluoride, silica, glycerin, water, sorbitol, PEG-8, Cocamidopropyl, xanthan gum, sodium methyl cocoyl taurate, sodium saccharin. The active ingredient in SRA is strontium acetate ions that interact with calcium ions and form strontium crystals within the dentinal tubules, resulting in the occlusion of the dentinal tubules, thus relieving dentin hypersensitivity [27]. Potassium nitrate is one of the key active ingredients in SRS that desensitizes sensitive tooth nerves by blocking the transmission of pain signals. Fluorides, on the other hand, strengthen tooth enamel. The method of action works by depolarizing nerves and stopping neural transmission. Fluoride can help the tooth enamel reclaim minerals7 that have been lost during the rotting process. When sodium fluoride is given to exposed dentine, it creates an effective barrier and causes dentine desensitization [28].
In our study, 13% percent of DH cases did not improve following the first line of treatment, and these cases had previously received orthodontic treatment and home bleaching. The existence of demineralized areas in the locations where the brackets and bands were installed produced DH [29]. Furthermore, bleaching has a solution that can dissolve minerals from the enamel and cause the teeth to become temporarily porous, exposing micro- tubules and causing DH [30]. In those circumstances, the in-office desensitizing agent GLUMA was used as a second line of treatment. GLUMA, a commercially available dentin desensitizer, is made up of glutaraldehyde, a low molecular weight and cross-linking material that reacts with serum albumin in the dentinal fluid to occlude dentinal tubules and reduce their diameter by coagulating and precipitating dentinal amino acids and proteins. GLUMA contains hydroxyethyl methacrylate (HEMA), a wetting agent that occludes dentinal tubules through a polymerization reaction. Its hydrophilic nature allows it to penetrate deep into dentinal tubules [31]. Previous studies also reported the effectiveness of GLUMA on DH [32].
Unlike other previous studies where the participants were from different educational and socioeconomic backgrounds, our study focused on participants who were well-versed in hypersensitivity and oral hygiene. This might be a contributing factor to obtaining a dissimilar etiology of DH compared to other studies. To date, there is no relevant information about the prevalence of DH among dental students, which is the novelty of the current study. At the same time, it considers the limitation of this study as we could not compare the prevalence in similar populations. Although the tested treatment protocol showed a good recovery from DH, the long-term recovery and recurrence of DH need to be studied further.
CONCLUSION
The prevalence of DH among the tested undergraduate dental students was 19.3%. The prevalence and the etiological factors for DH may differ among populations with different educational and socioeconomic back- grounds. The home-based desensitizing agent Sensodyne Rapid Action is effective in reducing DH. An in-office-based desensitizer like GLUMA is effective in reducing DH in patients who did not recover using a home-based desensitizing product.
LIST OF ABBREVIATIONS
| HEMA | = hydroxyethyl methacrylate |
| DH | = Dentin hypersensitivity |
| SRA | = Sensodyne Rapid Action |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research was approved by the Ministry of Health and Prevention UAE RAK-REC approval number MOHAP/REC/2021/ 58-2021-UG-D and the ethical committee of RAKMHSU-REC-025-2021/22-UG-D.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
The procedure was explained to the participants, and written consent was obtained prior to examining the participants.


