All published articles of this journal are available on ScienceDirect.
Nonsurgical Minimally Invasive Endodontic Treatment of Large Periapical Lesions: A Report of Three Cases
Abstract
Introduction
Periapical lesions develop as a result of microorganisms from necrotic pulp tissue or retained foreign bodies in the periapical tissue, leading to acute or chronic inflammation. It has been proven that the majority of inflammatory periapical lesions can be effectively treated using nonsurgical endodontic management. However, the selection between surgical and nonsurgical endodontic treatment for managing large periapical lesions remains a controversial issue. This report aims to reveal the effectiveness of nonsurgical, minimally invasive endodontic treatment for large periapical lesions in conjunction with lesion decompression and aspiration.
Case Presentation
This article presents three cases with large periapical lesions related to severe bone destruction with different causes that were managed with nonsurgical endodontic treatment, involving multiple visits for intracanal medication with calcium hydroxide. In all three cases, the long-term clinical assessments and CBCT scans consistently showed complete healing. The follow-up period ranged from 24 to 48 months.
Conclusion
The successful results demonstrated the efficacy of nonsurgical endodontic management, which is a minimally invasive approach when addressing large inflammatory periapical lesions with diverse causes.
1. INTRODUCTION
Periapical lesions that occur as a result of infection originating from necrotic pulp tissue, which can be caused by deep caries or trauma, are known as Lesions of Endodontic Origin (LEO). In the presence of bacteria and their byproducts, periapical tissues initiate an immune response, leading to the development of various types of periapical lesions, including granulomas, periapical cysts, or abscesses [1, 2]. The prevalence of these lesions varies among studies, with clinical evidence suggesting a higher prevalence of cystic lesions in cases with larger lesion sizes. However, large granulomas can also be observed. The final diagnosis of periapical lesions requires histopathological examination [3].
Treatment options for managing large periapical lesions encompass a spectrum ranging from nonsurgical endodontic treatment to surgical interventions, such as apicoectomy or tooth extraction. However, in alignment with minimally invasive endodontics, nonsurgical endodontic treatment is favored. The objective of this approach is to eliminate or reduce the bacterial load within the root canal system, thereby creating a conducive biological environment for the healing of periapical lesions [4]. The success of the healing process depends on the treatment protocol, including the selection of irrigation solutions and intracanal medicaments [5, 6]. These factors play a crucial role in modulating the host’s immune response, ultimately aiming to achieve the primary goals of endodontic treatment: eradication of periapical infection and prevention of periapical reinfection. The success rate of initial nonsurgical endodontic treatment has been reported to be as high as 97% [7]. However, treatment failures may occur, often attributed to the presence of residual bacteria or retained foreign bodies that trigger inflammatory and immune responses, leading to bone destruction in the periapical region [8]. Therefore, root-end resection and retrograde under the microscope have emerged as an alternative approach for managing large periapical lesions, offering more predictable treatment outcomes [9, 10]. The selection between surgical and nonsurgical endodontic treatment for managing large periapical lesions remains a controversial issue despite numerous studies demonstrating no significant difference in success rates [11, 12]. Nonetheless, the inclination towards conservative treatment is still favored by many clinicians when considering nonsurgical endodontic management [13].
This article presents successful nonsurgical endodontic treatment of large periapical lesions in 3 clinical cases using calcium hydroxide as canal dressing with a follow-up period ranging from 24 to 48 months.

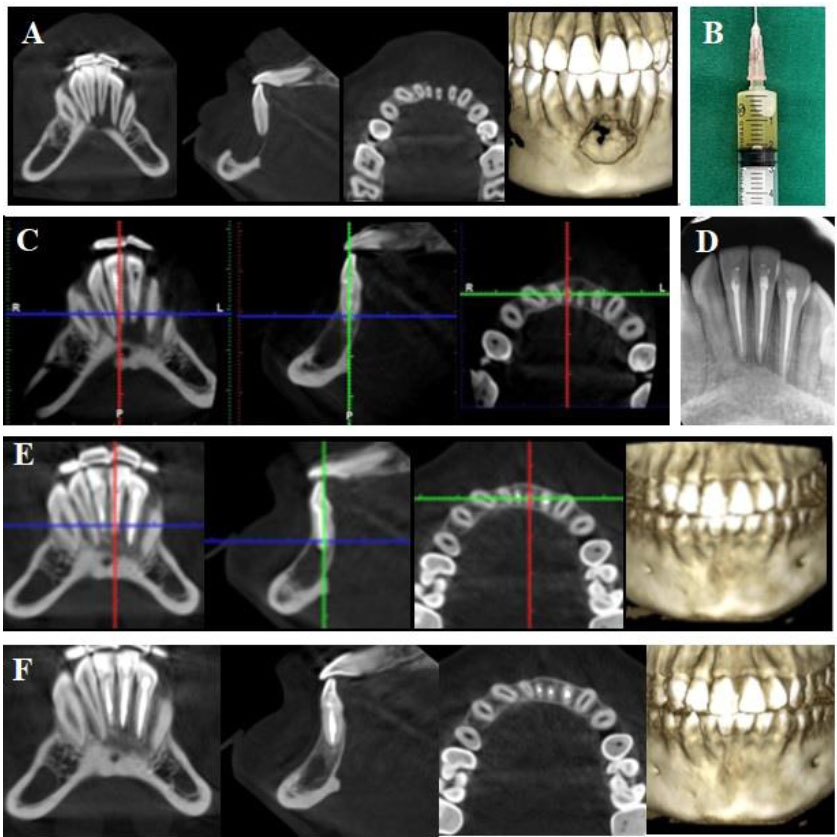
(A) Preoperative CBCT: a large periapical lesion associated destruction of cortical bone plates and an immature tooth #41; (B) Panoramic radiograph after first intervention by the general dentist; (C) Clinical image of lesion decompression with latex through buccal vestibule; (D) IOPA radiograph after root canal filling with MTA of tooth #41; (E) CBCT at 12-month follow-up; (F) CBCT at 36-month follow-up.
2. CASE PRESENTATION
2.1. Case 1
A 14-year-old male patient was referred to our hospital by a general dentist who had performed the root canal treatment for teeth #31, 32, and 42 and had planned for cyst removal surgery and extraction of tooth #41. Two days after root canal treatment, the patient reported severe pain and persistent swelling in the submental region and buccal vestibule. According to the dental record, the patient had been diagnosed with an infected radicular cyst related to lower incisors following occlusal trauma on tooth #41. The Cone-Beam Computerized Tomography (CBCT) showed a large, well-defined radiolucency involving mandibular incisors, causing deviation of their roots. The lesion extended beyond both the buccal and lingual cortical bone (Fig. 1A). Extraoral examination showed a diffused swelling in the submental region and tenderness upon palpation. Intraoral examination revealed vestibular swelling. Tooth #41 remained untreated, left opened and it was sensitive to percussion and palpation. Panoramic radiography showed a large lesion extending from tooth #32 to tooth #42, and tooth #41 was at the Cvek stage 3 of root development (Fig. 1B). Root canal treatment had been performed on teeth #32, 31, and 42. Based on these findings, the patient was diagnosed with submental cellulitis and previously treated symptomatic apical periodontitis on an immature necrotic tooth #41 due to occlusal trauma. The patient was indicated for intervention with nonsurgical endodontic treatment on tooth #41. In the first session of intervention, tooth #41 was irrigated with copious 3% sodium hypochlorite (Canal Pro 3%, Coltène/Whaledent GmbH + Co. KG, Germany) using 30G irrigation needle (Max-I-Probe Irrigation Probe, Dentsply Maillefer, North America), followed by ultrasonic activation (Irrisafe, Satelec Acteon, France) under rubber dam isolation. The working length was determined by radiography with a #10 K file (Dentsply Maillefer, Ballaigues, Switzerland). During the irrigation, straw-colored pus continuously came out through the access cavity. Intraoral drainage was established through the buccal vestibule using latex for 24 hours, and tooth #41 was filled with calcium hydroxide (Ultracal, Ultradent Inc., Utah, USA) and coronally sealed with temporary filling material (Fig. 1C). Amoxicillin (2g per day) was prescribed for 5 days. After 24 hours, there was a decrease in the patient’s pain and swelling severity, so the latex tube was removed. Three weeks later, the canal was irrigated with 3% sodium hypochlorite, and calcium hydroxide was reintroduced into the canal. The patient became asymptomatic after 3 weeks. At the third appointment, the copious irrigation with 3% sodium hypochlorite activated with ultrasonics was carried out, followed by EDTA 17% for 1 minute and final rinse with 3% sodium hypochlorite. The canal was dried and obturated with mineral trioxide aggregate (ProRoot MTA, Dentsply Tulsa Dental Specialties, USA) (Fig. 1D). Coronal restoration was achieved using composite (Tokuyama Dental Corp., Tokyo, Japan). The patient was reexamined clinically and radiographically after 12, 24, and 36 months. The patient remained consistently asymptomatic. CBCT at 12 and 36 months postoperative revealed complete healing with bone regeneration, and the roots of the incisors returned to the original position (Figs. 1E and 1F).
2.2. Case 2
A 13-year-old female patient was referred to our hospital due to symptoms related to a chin injury sustained twelve months prior. The patient exhibited swelling, erythema, heat, tenderness, and induration in the chin area extraorally. Intraoral examination revealed swelling and tenderness in the vestibule and grade 3 mobility of teeth #32, 31, and 41, with severe sensitivity to percussion and palpation. Teeth #32, 31, and 41 were unresponsive to cold and electric pulp tests, while tooth #42 had a normal response. CBCT revealed a sustained radiolucency associated with teeth #32, 31, and 41, along with evident destruction of the buccal cortical plate (Fig. 2A). Based on these findings, the patient was diagnosed with necrotic pulp and acute apical abscess in teeth #32, 31, and 41 [14]. Nonsurgical endodontic treatment was indicated. During the first visit, the lesion was aspirated with a sterilized syringe through the vestibule, and the teeth were accessed under rubber dam isolation. Yellowish fluid was drained through the cavity (Fig. 2B). After discontinuation of the drainage, the working length was determined using an electronic apex locator (ProPex II, Dentsply Maillefer, Ballaigues, Switzerland). Cleaning and shaping of the canal were performed using the reciprocating file (WaveOne Gold, Dentsply Maillefer, Ballaigues, Switzerland) and 3% sodium hypochlorite with ultrasonic agitation. The canals were then dried and filled with calcium hydroxide. The access cavity was sealed with temporary filling material for 3 weeks. The patient was instructed to take Amoxicillin, 2 grams per day for 5 days, and subsequently reported no pain and a significant reduction of swelling at the chin region. Intraoral examination revealed the absence of mobility, sensitivity to percussion, or palpation of teeth #32, 31, and 41. The calcium hydroxide dressing was repeated three more times, each for 3 weeks. After 12 weeks, the patient was asymptomatic, and a buccal cortical plate was observed on CBCT (Fig. 2C). All canals were irrigated with 3% sodium hypochlorite under rubber dam isolation, followed by 17% EDTA (Canal Pro, Coltène/Whaledent GmbH + Co. KG, Germany) then finally rinsed with 3% sodium hypochlorite. All irrigants were introduced into the canals with a 30G needle. The root canal system was obturated using the wave continuous condensation technique with the warm vertical compaction system (Elements Obturation, SybronEndo/Kerr Endodontics, Orange, CA) (Fig. 2D). The 12 and 24-month follow-up evaluations presented no tenderness to palpation and percussion, and CBCT showed steady bone regeneration (Figs. 2E and 2F).
2.3. Case 3
A 33-year-old female patient presented to our hospital with a chief complaint of pain and swelling in the buccal vestibule area. She had been advised to undergo surgery, including extraction of tooth #46 and cyst enucleation. However, seeking a more conservative treatment approach, the patient visited our hospital. Intraoral examination revealed swelling in the buccal vestibule and tenderness upon palpation. Tooth #45 was covered with a ceramic fused to a metal crown and exhibited sensitivity to percussion and palpation. Meanwhile, tooth #46 showed a normal response to cold and electric pulp tests. Radiographic examination showed a large radiolucency associated with both teeth #46 and #45 (Fig. 3A), with tooth #45 having undergone a previous root canal procedure. The patient was diagnosed with previously treated, symptomatic apical periodontitis on tooth #45. Nonsurgical root canal retreatment was indicated for tooth #45. During the first appointment, the crown of tooth #45 was removed, and a rubber dam was placed. The gutta-percha was removed using rotary files (Protaper D1-D3, Dentsply Maillefer, Ballaigues, Switzerland), and copious irrigation with 3% sodium hypochlorite was performed under the activation of ultrasonics. After the complete removal of the gutta-percha, the working length was determined using the ProPex II apex locator. The canal was then reshaped by a reciprocating file (WaveOne Gold, Dentsply Maillefer, Ballaigues, Switzerland) and medicated with calcium hydroxide for a three-week interval. In the subsequent appointment, the patient was asymptomatic, and disinfection and obturation of the canals were done similarly to case 2 described above (Fig. 3B). Clinical and radiographic assessments were conducted at 12, 24, 36, and 48 months, with CBCT imaging showing successful healing in which the complete healing obtained at 48-month recall periods (Figs. 3C and 3D). Tooth #46 exhibited a positive response to cold and electric pulp tests.
(A): Preoperative CBCT: a large periapical lesion with destruction of the buccal cortical bone plate; (B) fluid aspirated from the lesion; (C) CBCT after 12-week intracanal medication with calcium hydroxide; (D) IOPA radiograph after root canal filling of teeth #32, 31, and 41. (E) CBCT at 12-month follow-up; (F) CBCT at 24-month follow-up.
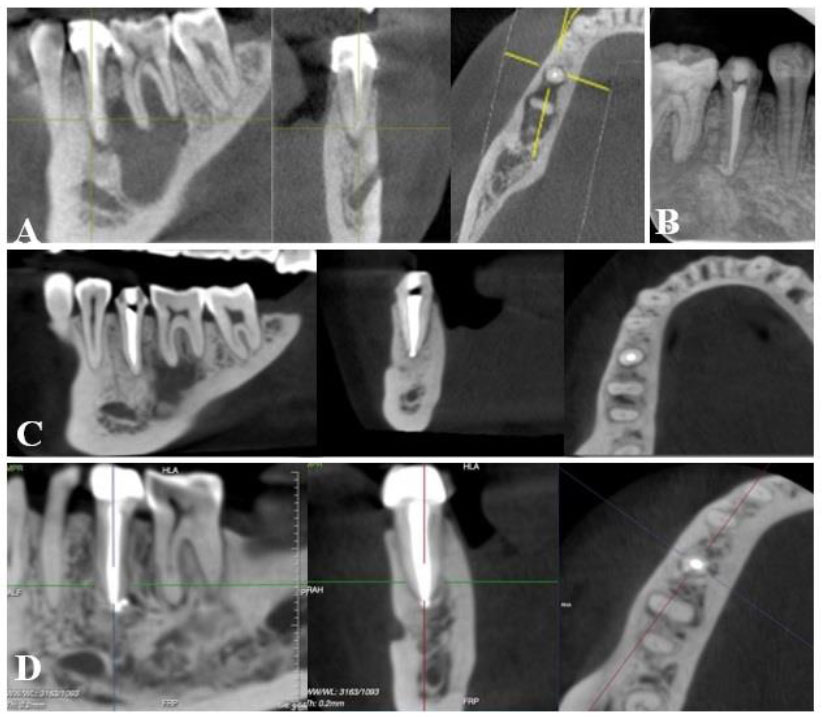
(A): Preoperative CBCT: a large periapical lesion associated with tooth #45 and #46; (B) IOPA radiograph after root canal retreatment of tooth 45; (C) CBCT of 12-month follow-up showed healing of periapical lesion with a remaining radiolucency around the mesial root of tooth #46; (D) CBCT at 48-month follow-up, showed complete healing.
3. DISCUSSION
The histopathology study of Ricucci et al. (2006) reported that periapical lesions consist of 40% granulomas, 32% periapical cysts, and 28% abscesses. They are typically caused by intraradicular or extraradicular infections [15]. Bacteria originating from the pulp tissue or their remnant in the root canal after treatment play a crucial role in this process [2]. Recent studies found a high prevalence of bacterial biofilms in the root canals, even in treated teeth with apical periodontitis, particularly in cases with large lesions and cysts [16]. Marina Fernandes et al. (2010) stated that a nonsurgical approach should always be adopted before considering surgery, and the success rate reached as high as 97% [7, 17]. The primary objectives of endodontic treatment, as reported by Shilder H., are to thoroughly clean, shape, and seal the root canal system in three dimensions. This approach aims to effectively treat or prevent apical periodontitis and preserve natural teeth [18, 19]. The presented cases in this article exhibited large periapical lesions caused by various factors, including occlusal trauma, dental injury, and failed endodontic treatment. The first two cases involved microorganisms from necrotic pulp as a result of trauma, leading to periapical infections and subsequent bone destruction. The last case involved an unsuccessful root canal treatment on a premolar, resulting in a large periapical lesion that extended toward the first molar. In cases with large periapical lesions, accurately identifying the responsible tooth using pulp tests is essential to eliminate the source of microorganisms and their byproducts.
In these cases, the irrigation protocol featured the use of abundant 3% sodium hypochlorite activated by ultrasonic agitation, followed by 17% EDTA to eliminate the smear layer. A systematic review and meta-analysis of randomized controlled trials conducted by Kasidid Ruksakiet et al. (2020) found that sodium hypochlorite significantly, but not completely, mechanically and chemically eliminated endodontic infections during root canal therapy [20]. However, it is considered a unique solution that can dissolve necrotic tissue [21]. Many studies have revealed that the efficacy of sodium hypochlorite on endodontic biofilm is accelerated with ultrasonic agitation, and other researchers have reported that ultrasonic activation of irrigants improves debridement, disinfection, and smear layer removal, resulting in better cohesion between the sealers and the dentin tubules, preventing apical leakage and tooth fracture [22-27]. The use of ultrasonic agitation in case 1 can be considered a minimally invasive shaping method for an immature necrotic tooth #41 [28]. In our case 1 and case 2, decompression of the lesion through the vestibule (case 1) and aspiration of the lesion through the root canal (case 2) helped relieve pain and reduce fluid pressure inside the periapical lesion. Marina Fernandes et al. (2010) concluded in their study that decompression and aspiration-irrigation techniques decrease the hydrostatic pressure of the lesions and facilitate drying of the canal for intracanal medication with calcium hydroxide [17]. In all three cases, calcium hydroxide was used as an interappointment intracanal medication to enhance root canal disinfection by targeting the remaining bacteria that cannot be eliminated by chemomechanical irrigation [29]. Based on its broad bactericidal effects and high pH, calcium hydroxide creates a consistently favorable condition for periapical healing when placed intracanal for less than 28 days [30, 31]. However, Doyon, Glen E et al. (2005) reported that long-term exposure of the dentinal wall to calcium hydroxide decreases the fracture resistance of the root, so the interval time for calcium hydroxide in these cases was three weeks [32, 33].
In these three cases, periodic clinical examination and radiographic testing were performed with a 12-month follow-up interval. The CBCT scans showed the absence of periapical lesions after 12 months in all three cases. The long-term results showed complete healing after 36 months in case 1, 24 months in case 2, and 48 months in case 3. Following the guidelines of the European Society of Endodontology, the first recall should be at 12 months, and further evaluation should be done after 4 years if the results are questionable [34]. In the first case, the pulp of tooth #41 was necrotic due to occlusal trauma, leading to an immature tooth and a large periapical lesion associated with other lower incisors in the CBCT. The treatment option for this case was a full root canal filled with MTA, creating a calcified barrier at the apex region [35]. Pace R. et al. (2014), in a report of a 10-year case series, showed that MTA was an effective technique for the long-term management of immature necrotic teeth with large periapical lesions [36]. Case 2 and case 3 were managed by conventional nonsurgical endodontic treatment/ retreatment, which resulted in successful healing after 12 months (case 2) and 48 months (case 3). The healing process of the periradicular tissue after the endodontic infection is completely controlled by nonsurgical root canal treatment, and the tissue healing is influenced by multiple host and treatment factors [37]. The periapical tissue regenerates from the periphery to the center of the lesion, as observed in the CBCT throughout the recall time in these presented cases [38, 39].
CONCLUSION
Three cases presented in this article exemplify the effective treatment of large periradicular lesions utilizing calcium hydroxide intracanal paste with nonsurgical root canal therapy. While this report was limited to three cases, complete healing was achieved through a minimally invasive strategy involving nonsurgical endodontic intervention targeted at the causative teeth. These findings contribute to the existing evidence confirming the efficacy of nonsurgical endodontic management in addressing extensive inflammatory periapical lesions arising from various origins.
ABBREVIATIONS
| LEO | = Lesions of Endodontic Origin |
| CBCT | = Cone Beam Computerized Tomography |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of the National Hospital of Odonto-Stomatology with No. 606/QD-BVRHMTW.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.


