All published articles of this journal are available on ScienceDirect.
Histological Study to Evaluate the Effect of Local Application of Myrtus Communis Oil on Alveolar Bone Healing in Rats
Abstract
Background
Reproving dental defects is still a significant problem in dentistry. Bone is a highly vascularized tissue that is reliant on maintaining skeletal structure. The medicinal properties of healthy and preventative herbs were recognized by both the ancient and modern pharmacists and doctors in medicine. The effects of Myrtus communis oil (M) are employed as a healing agent for bone loss with anti-inflammatory and antioxidant properties. The advantage of Myrtus communis oil (M) is that it is a form of osteoconduction in the process of bone healing, decreases pain, and decreases the length of time needed for bone healing. Aim: to identify the effectiveness of Myrtus communis oil's local application in healing bone defects; the author performed a histological analysis.
Materials and Methods
This study involved 12 albino male rats weighing (300-400) grams, aged (6-8) months. The animals were subject to a surgical operation on the alveolar bone. The group of animals was divided into two distinct categories based on the applicable materials. Control group: 6 rats; bone defect was only washed by normal saline, and bone defect was left to heal normally. Myrtus communis group: 6 rats; bone defect treated by local application of 1 μl of Myrtus communis oil (M). The rats were sacrificed 7 and 14 days after surgery (six rats for each period). All bone sections stained with hematoxylin and eosin underwent a light microscope histological inspection, which included counting the number of bone cells (osteoblasts, osteocytes, and osteoclasts) and evaluating the results of histomorphometric analysis.
Results
Histological and histomorphometric findings of the present study show the acceleration of bone defect healing process in the Myrtus communis group as the increase in mean count difference measured of osteoblast, osteocyte, and osteoclast with statistically significant in 7 days duration. The result shows the positive expression of osteoblast, osteocyte, and osteoclast in all groups, with the highest statistical difference in osteocyte mean number in the Myrtus communis group.
Conclusion
Myrtus communis oil (M) has the potential to promote and be an effective therapeutic for the bone injury healing process.
1. INTRODUCTION
Bones are solid organs that participate in the endoskeleton of animals with a backbone. They assist and defend the various body organs, produce red and white cells of the blood and store minerals [1].
Common trauma injuries to the skeletal system are called bone defects, and their repair and healing processes include an inflammatory response, the recruitment of stromal progenitor cells, the proliferation, differentiation, and maturation of chondrogenic or osteoblastic cells, as well as extensive matrix production and remodelling [2].
The osseous healing process relies on four key components: the cortex, periosteum, bone marrow, and the surrounding soft tissues [3]. Bone healing, modelling and remodelling are regulated by hormones and local factors [4].
Traditional herbal healing accounts for approximately 80% of primary healthcare in many medical systems, but there are no studies on the long-term effects of single-dose local application of herbal oils [5, 6].
The Myrtaceae family includes Myrtus communis L, one of the most widely used medicinal herbs. Documentation of the use of botanical chemicals in the Unani medical system dates back to ancient Greece. The plant's essential oil and various parts have been used for several purposes for a long time. Various purposes include cosmetics (controlling hair loss), flavouring food and drink, enhancing health—drinks, and extensive therapeutic purposes. Ethnicity-specific knowledge revealed that M. communis L. has traditionally been used as a folkloric remedy for gastric ulcers, diarrhoea, dysentery, cancer, rheumatism, and obesity. Loss of blood, severe pain, fatigue, shortness of breath, chest pains, and other symptoms associated with the flu. Anxiety, insomnia, diabetes, hypertension, pulmonary issues, and skin diseases [7].
Numerous ethnopharmacological examinations have exhibited the vast pharmacological potential of this plant, which includes but is not limited to its ability to act as an antimicrobial, antidiarrheal, antidiabetic, antispasmodic, vasodilator, antiulcer, antioxidant, anticancer, anxiolytic, sedative-hypnotic, and anti-inflammatory agent. In addition, the plant is well known to contain phenolic acids, tannins, flavonoids, glycosides, and terpenes. Further more, the myrtle oil extracted from the plant was discovered to be abundant in an array of biologically active monoterpenes and sesquiterpenes, along with their derivatives. These studies corroborate the traditional medicinal claims linked to this plant. However, additional research must be performed to uncover other potential pharmacological applications of this plant in the future [8].
2. MATERIALS AND METHODS
2.1. Study Design
Every experimental procedure strictly adhered to the established ethical principles of animal experimentation Local Ethical Committee (Reference number 5718). In this experimental study, 12 albino rats, weighing (300-400) grams, aged (6-8) months were food consumption. The animals were subjected to the surgical operation of alveolar Bone.
2.2. Supplement of Plant Extracts
The super concentrated liquid herbal extract contains no alcohol, no additives materials and is manufactured in an FDA was sourced from certified vendors (Hawah Pharm, Gilbert, AZ, USA)
2.3. Sample Size
The research's sample size was determined using the mean values of biomechanical assessment data obtained from a prior study [9]. With a significance level (α) of 0.05 and a confidence interval of 95%, the power was determined to be 95%. The effect size was calculated to be 0.318, and the sample size was determined to be 8 rats per group using G-power software version 3.1.90. A total of 6 rats were assigned to each group for the purpose of this investigation. Twelve rats were subjected to a surgical operation of the buccal sides of the alveolar bone in the mandible (the right side was considered) as the experimental side.
The animals were divided into the following groups:
A. The control group contained six rats. The bone defect was left to heal spontaneously in a normal way.
B.Experimental group include
Group M contains six rats; the bone defect was treated with 1 μl of (M)
Every single group composed of 6 rats that studied in two periods 7, 14 days (3 rats for each period).
2.4. Study Hypothesis
2.4.1. Null Hypothesis
There are no statistically significant differences in the effectiveness of using (M) in an application on bone defect (osteocyte, osteoblast and osteoclast).
2.4.2. Alternative Hypothesis
There are statistically significant differences in the effectiveness of using (M) in an application to bone defects (osteocyte, osteoblast, and osteoclast).
| Drug | Dose | Route | Duration |
|---|---|---|---|
| Anaesthetics | - | - | - |
| Pentobarbital | 30-60mg/kg | IP | 20-60 min |
| EMTU(inactin) | 80-100mg/kg | IP | 60-240 min |
| Dissociative agents and combination | |||
| Ketamine+ | 75 mg/kg | IP | 20-30 min |
| Acepromazine | 2.5 mg/kg | - | - |
| Ketamine+ | 60-75 mg/kg | IP | 20-30 min |
| Medetomidine | 0.5 mg/kg | - | - |
| Ketamine+ | 40-80 mg/kg | IP | 20-40 min |
| Xylazine | 5-10 mg/kg | - | - |
| Ketamine+ | 40-50 mg/kg | IP | 20-30 min |
| Xylazine+ | 2.5-8 mg/kg | - | - |
| Acepromazine | 0.75-4 mg/kg | - | - |
2.5. Sample Housing
The rats were housed in separate cages in a 12:12 light/dark environment at a constant humidity and temperature of (23 °C) during the experimental study period, which began on January 5, 2023, and ended on March 5, 2023, in the animal department of the veterinary medicine college at Kufa University / Najaf - IRAQ. This was done by the guidelines provided by the National Research Council, which outlines the proper care and utilization of laboratory animals. The group and number of each rat were used to identify the cage number given a regular diet consisting of pellets and water on demand, which is renewed daily.
2.6. Methods
2.6.1. Instruments Sterilization
In surest to obtain maximum sterilization of all the instruments used in surgical operation, they were sterilized in the oven at 150 °C for 1 hour, and then the instruments were mantled with the aluminium foils and sterilized in the autoclave at (150 °C) and (15 bar/cm2) also for 1 hour (10).
2.6.2. Surgical Procedure Technique
Ketamine hydrochloride 10% (50 mg/kg body weight) and xylazine 2% (5 mg/kg body weight) were injected intramuscularly during the surgical procedure while under general anaesthesia (11) (Table 1).
-The procedure was performed using an elegant surgical technique and in the necessary sterilized conditions.
-A skin incision was made using a sharp blade (no. 10), exposing the mandible and reflecting the skin and fascia flap.
-A bone defect hole of 1.8 mm was made using a small round bur, intermittent drilling, and continuous cooling and irrigation with regular saline (1).
-To clear the drilling site of debris, saline solution was used to wash the operation site. On the mandibular Bone, a bone defect developed.
-Wash the region with regular saline after the procedure for the control group. As the region was being dried by air in the experimental groups, (M) was used to apply the material according to each group.
- The amount of oil applied to the rats was 1 μl, and it was determined based on the study design. The oil was applied via a micropipette.
-After applying Myrtus communis using a micropipette, a 3/0 absorbable (catgut) suture was used to close the wound.
-Following the procedure, the animals were given a 20% (0.7 mL/1kg) dose of the long-acting systemic antibiotic oxytetracycline. An antibiotic was administered systemically as part of the post-operative therapy. Therefore, there was no weight loss or infections in the rats after the operation. Using an overdose of anaesthesia, the animals were sacrificed at intervals of seven and fourteen. At the surgical location, skin, facia, and muscles were removed.
-To prevent bone injury, bone specimens were prepared by cutting the Bone approximately 5 mm away from the operation site and continuously rinsing it with saline six animals per group, three for each period. After dissecting, the mandible bone was preserved in 10% buffered formalin.
2.6.3. Tissue Processing and Staining for Histological Examination
2.6.3.1. Method
1. Fixation. Made three days' worth of formalin.
2. Decalcification. The 10% formic acid solution in which the specimens were kept was replaced every three days.
The Chemical Test was used to determine whether the Bone had decalcified.
To create the Ammonium Hydroxide/Ammonium Oxalate Working Solution, one must combine equal portions of two ingredients. These are the 5% Ammonium Hydroxide Stock and the 5% Ammonium Oxalate [10-12].
3. Using tap water to wash the samples.
4. Loss of moisture. Following a sequence of escalating alcohol concentrations (40%, 60%, 80%, 95%, and 100% alcohol), the specimens were dehydrated. Subsequently, the samples were run through two xylene jars, one for thirty minutes each.
5. Integration. The samples were put in a plate with melted embedding paraffin and then heated in an oven set to a consistent temperature of between 53 °C and 60 °C.
6. The rat mandible was sectioned into five μm thick sections, and the sections were then placed on sanitized glass slides for standard H&E staining [13].
2.6.3.2. Hematoxylin and Eosin Stain
1. The obtained sections were rehydrated in descending alcohol concentration after being dewaxed with xylene.
2. Hemotoxylin staining took seven to ten minutes.
3. To remove extra discolouration, wash in tap water for one to five minutes.
4. It was then given a one to two-minute eosin stain.
5. Dehydrated in 100% alcohol for two to three minutes, then wiped with xylene.
6. Using D.P.X., cover slips were adhered to dyed tissues.
7. Histological examination of slides was done under the light microscope [14].
2.7. Histomorphometric Analysis for Bone Microarchitecture
Measurement was conducted on the microarchi- tectures listed below.
The process of counting bone cells involves the quantification of osteoblast cells per square millimetre (OB/mm2).
The numerical representation for the number of osteocyte cells per square millimetre is denoted by OC/mm2.
The numerical value represents the quantity of osteoclast cells present within a specific area, measured in units of OCL per square millimetre [12, 13].
It was performed by counting bone cells in H&E stained histological sections in five microscopic fields at x40 magnification at two healing periods, 7 and 14 days.
2.8. Statistical Analysis
The study's findings were examined and evaluated using the following statistical methods for data analysis:
2.8.1. Descriptive Data Analysis
Mean value, Standard Deviation, Standard Error, (95%)
Confidence interval for population Mean values, two Extreme values (min. and max.).
2.8.2. Inferential Data Analysis
These were used to accept or reject the statistical hypotheses, which comprised the following:
1. F-test for equality of means for coincidence testing in samples.
2. T-test (LSD test) for less significant differences in the means of different independent groups.
3. ANOVA, or general linear model, for two or three variables.
RESULTS
3.1. Histological Findings (Hematoxylin and Eosin Stain)
3.1.1. Control
3.1.1.1. At 7 Days Duration
After seven days, developing osteoblasts are seen at the rims of the bone matrix surrounding the osteocytes, fat cells, inflammatory cells, blood vessels, and adjacent progenitor cells, according to a histological analysis of the defect region in the control group. Figs. (1 and 2).

View of 7 days of the control group shows new bone matrix (black arrows), osteocytes (green arrows), fat cells (blue arrows), and inflammatory cells (yellow arrows). H&E X20.
3.2. Experimental Groups
3.2.1. Bone Treated with Myrtus Communis
3.2.1.1. At 7 Days Duration
After seven days, a histological examination of the defect area in the (M) group reveals trabeculated Bone that has merged with basal Bone and osteoid tissue; Figs. (5 and 6).
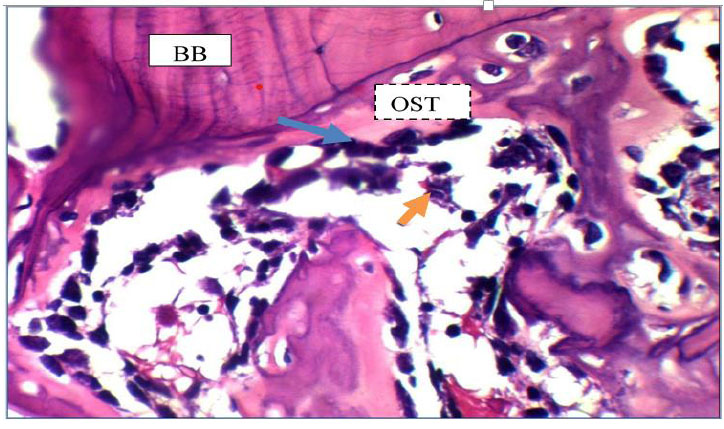
The view of the defect site of the control group after seven days shows basal Bone (BB), osteoid tissue (OST), osteoblasts (blue arrow), and reticular cells (yellow arrows). H&E X40.
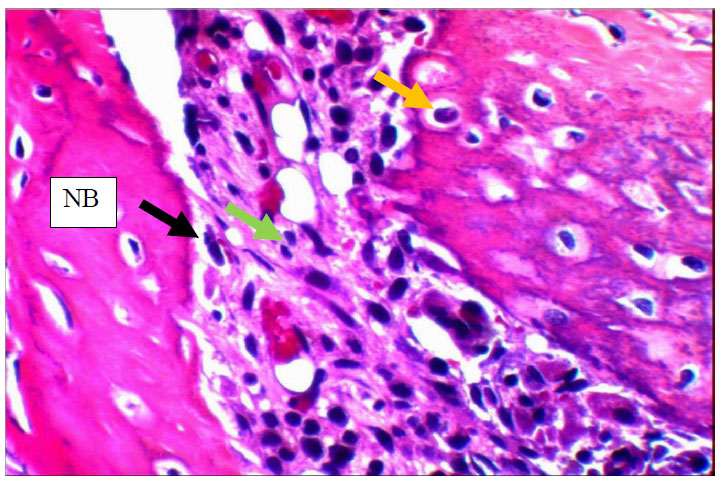
View of the 14days control group shows numerous osteocytes (yellow arrows) entrapped in new Bone (NB) that is rimmed by osteoblasts (black arrow) and shows inflammatory cells (green arrows).H&E x40.
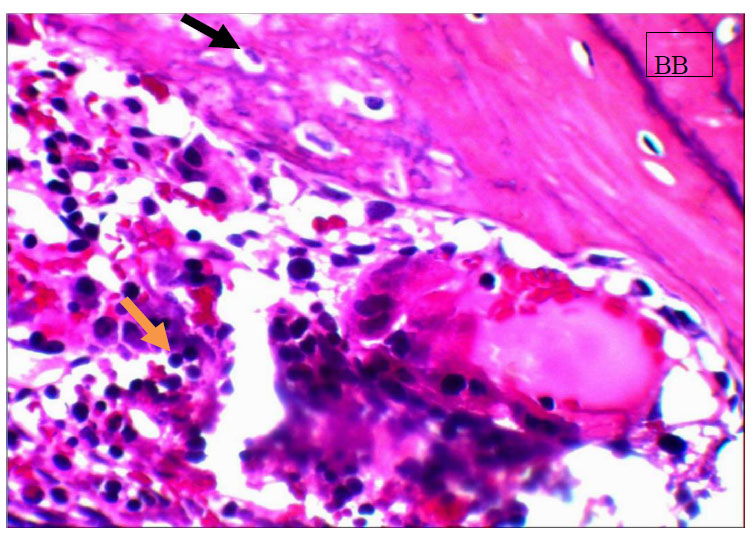
View for basal bone (BB), osteocytes (black arrow) entrapped in bone, inflamatory cells (yellow arrows) in the control group for 14 days. H&EX20.
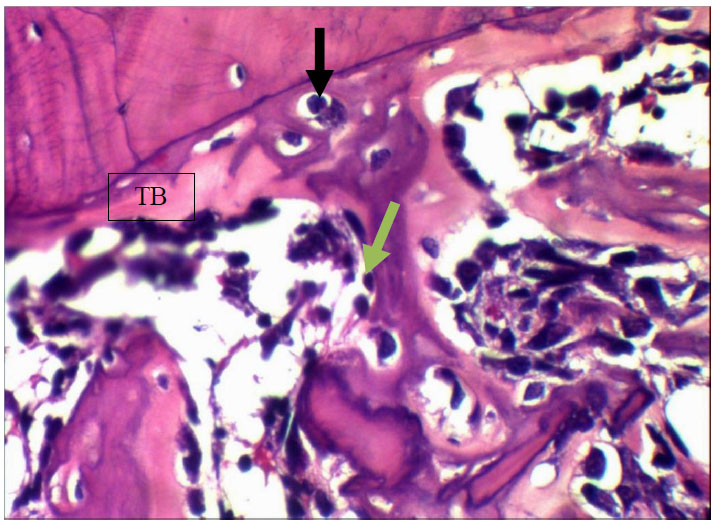
View after 7 days Myrtus communis application shows trabecular bone (TB) enclosing osteocytes (black arrow) and rimmed by osteoblasts (green arrows). H&EX40.
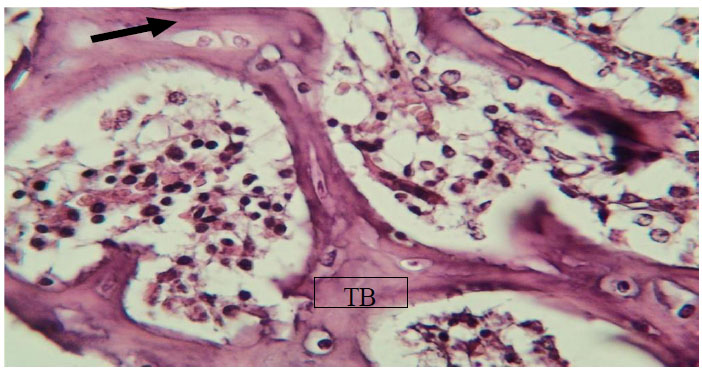
View after 7 days Myrtus communis application shows trabecular bone (TB) enclosing and osteocytes (arrow). H&EX40.
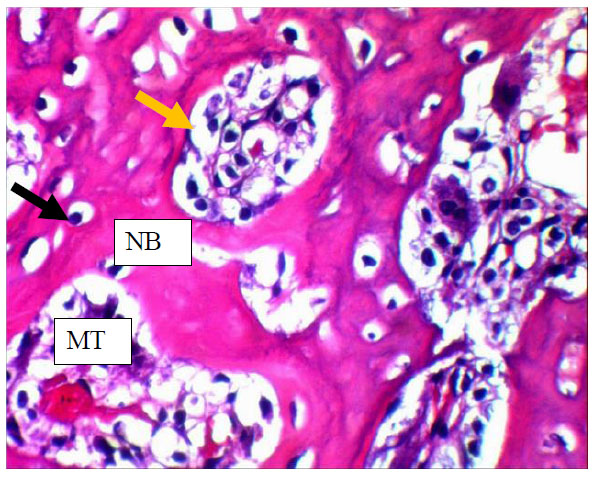
View of 14 days Myrtus communis group shows numerous osteocytes (black arrow) entrapped in new Bone (NB) rimed by osteoblast (yellow arrow) that encloses marrow tissue(MT) .H&EX40.
View of Myrtus communis group at 14 days shows osteocyte cells (arrows) entrapped in new Bone (NB) with marrow tissue (MT) inside it. H&E X20.
3.3. Statistical Analysis of Histological Findings (Hematoxylin and Eosin Stain)
3.3.1. Histomorphometric Analysis of Studied Groups for Bone Architecture Parameters
All research groups had their bone cell count statistics (osteoblasts, osteocytes, and osteoclasts) measured during varying periods (7 and 14 days).
3.3.1.1. Descriptive Statistics Analysis of Bone Cells
Descriptive statistical analysis for the mean, standard deviation, standard error, and minimum and maximum value of bone cell count, including osteoblast, osteocyte and osteoclast, is estimated in different groups and duration of 7-14 days (Table 2 and Fig. 9).
The osteoblast mean value (14.00) was recorded as highest in the M group in 7 days duration while the lowest was in the control group. The osteoblast number decreased in 14 days duration in all groups.
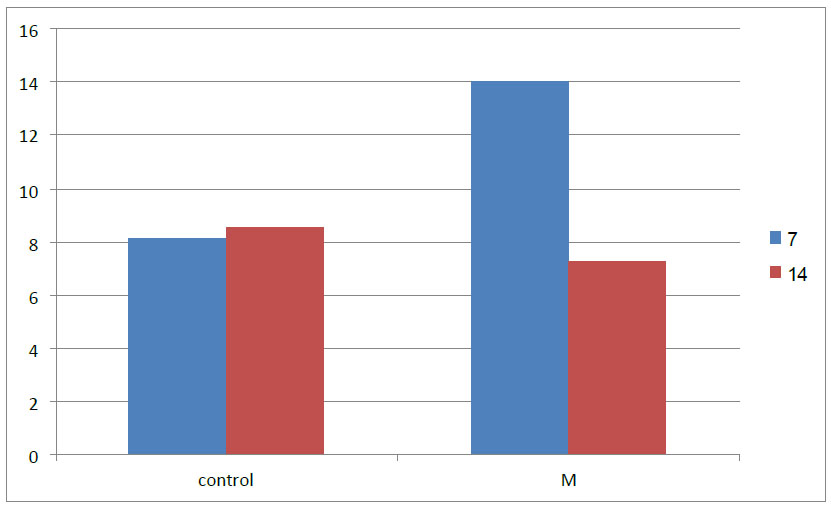
Comparison of the osteoblast mean at 7 and 14 days between research groups.
| Cells of Bone | Time | Items of Groups | D. statistic |
Group Difference |
||||
|---|---|---|---|---|---|---|---|---|
| M(mean) | S.Deviation | Minimum | Maximum | F__test | P__value | |||
| O.blast | Seven Days | Control | 8.10 | 1.544 | 5.00 | 8.50 | 36.00 | 0.000 (HS)* |
| M | 14.00 | 1.623 | 6.00 | 10.00 | ||||
| O.blast | 14 Days | Control | 8.54 | 0.822 | 8.33 | 10.10 | 3.246 | 0.049 (S) |
| M | 7.20 | 1.265 | 12.50 | 16.50 | ||||
| O.Cyte | Seven Days | Control | 9.84 | 0.922 | 5.25 | 8.45 | 15.28 | 0.000 (HS)* |
| M | 14.80 | 2.467 | 8.25 | 15.00 | ||||
| O.Cyte | 14 Days | Control | 7.54 | 1.418 | 8.50 | 12.00 | 8.62 | 0.000 (HS)* |
| M | 10.35 | 1.483 | 11.50 | 15.50 | ||||
| O.clast | Seven Days | Control | 0.35 | 0.335 | 0.35 | 1.10 | 1.45 | 0.005 (HS) * |
| M | 0.48 | 0.211 | 1.11 | 1.55 | ||||
| O.clast | 14 Days | Control | 0.64 | 0.641 | 0.86 | 3.11 | 9.98 | 0.001 (HS)* |
| M | 1.40 | 0.431 | 0.01 | 0.75 | ||||
Note: (S) Sagnificant ; (HS)* High significant.
Osteocyte mean value was recorded as highest in the M group (14.80 and 10.35) in 7 days and 14 days duration, respectively, while the lowest osteocyte mean value was in the control group(9.84 and 7.54), respectively.
Regarding osteoclast, the mean value registered as highest in the M group (0.48) and lowest in the control group (0.25) in 7 days duration. On the other hand, osteoclast number increased in number in 14 days in all groups, with the highest mean value in the M group (1.40) and lowest in the control group (0.55).
The data illustrates a highly significant value in the mean of all bone cells in different groups in each duration of 7-14 days except in osteocyte 14 days significant (S).
3.3.1.2. Group and Duration Differences by LSD Test of Bone Cells
Table 3 and Fig. (10) represents an LSD test that compares groups in individual periods of bone cells (osteoblast, osteocyte and osteoclast). The study groups show that osteoblast counts at seven days were recorded to be highly significant difference values, While it shows that (the osteocyte) count at seven days was recorded to be highly significant. In osteoclasts, a number expressed the same comparison result as in osteoblasts.
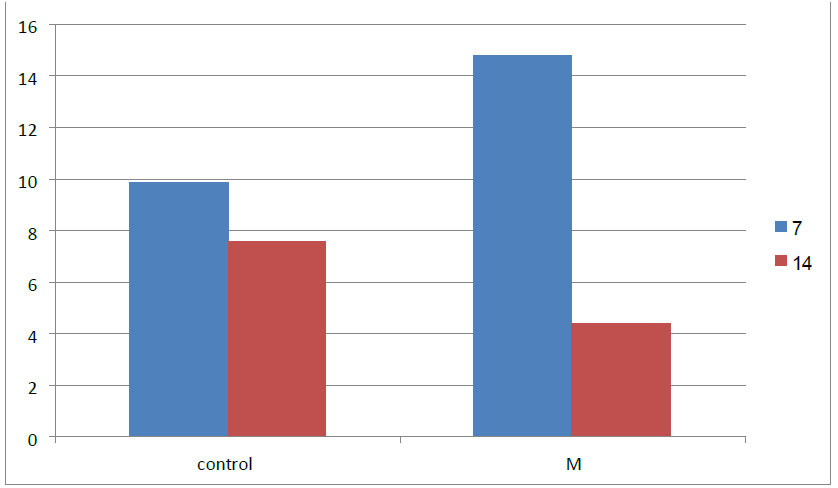
Comparison of the osteocyte mean at 7 and 14 days between research groups.
| Bone Cells | Duration | Groups |
Mean Difference |
P value | |
|---|---|---|---|---|---|
| O.blast | Seven days | Control | M | 6.75 | 0.000(HS)* |
| O.cyte | Seven days | Control | M | 3.840 | 0.000(HS)* |
| O.clast | Seven days | Control | M | 0.100 | 0.002(HS)* |
Note: (S) Sagnificant ; (HS)* High significant.
| Bone Cells | Duration | Groups |
Mean Difference |
P value | ||
|---|---|---|---|---|---|---|
| O.blast | Day 14 | Control | - | M | 1.10 | 0.027(S) |
| O.cyte | Day 14 | Control | - | M | 3.950 | 0.000(HS)* |
| O.clast | Day 14 | Control | - | M | 0.300 | 0.O33(S) |
Note: (S) Sagnificant ; (HS)* High significant.
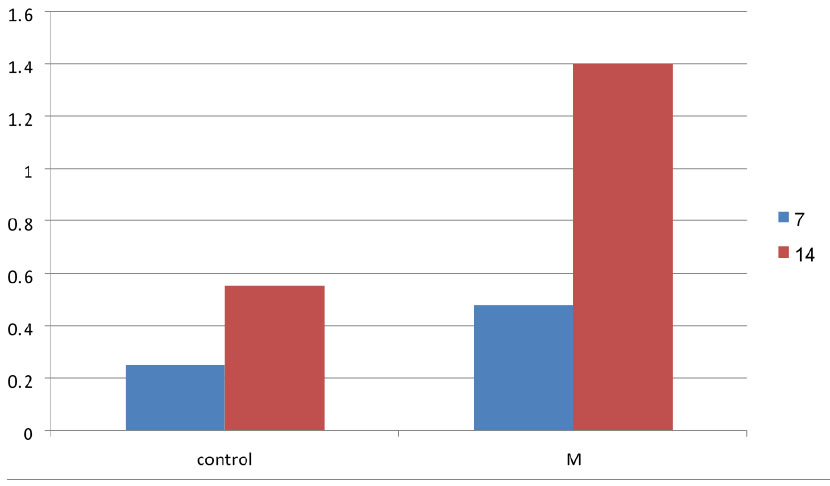
Comparison of the osteoclast mean at 7 and 14 days between research groups.
Table 4 and Fig. (11) represents the study groups, showing that (osteoblast, osteocyte, and osteoclast) count at 14 days records to be highly significant difference values.
4. DISCUSSION
The method of bone repair is greatly influenced by intricate interactions between cells of the mesenchymal stem cell-osteoblast lineage, specifically the monocyte, macrophage, and osteoclast cells. The latter lineage serves as a source of cells for immune regulation and bone regeneration, while the former controls inflammatory processes and bone resorption. Numerous issues, such as fracture healing, osteoporosis, diabetes, the elderly, and other connected events, may be helped by novel treatment strategies that affect inflammation and bone regeneration [15].
Herbal or traditional medicines have long played a significant role in the healthcare system. The pharmaceutical industry now views traditional medicine as a resource for finding bioactive ingredients that can be utilized to create synthetic medications [16, 17].
Myrtle oil has bio-active properties such as anti-inflammatory, antimicrobial and antioxidant effects. Depending on the long web search over the last few years, almost no study has been found that was concerned with the local use of (M) in accelerated bone healing. There are not enough articles in this thesis that discuss the same title.
4.1. Experimental Animal Description
Adult rats provide many desired characteristics; therefore, they were selected to fulfil the requirements of the study. The rat is the second most commonly utilized animal in biomedical and behavioural research, surpassed only by the mouse. The rat is considered an important laboratory animal due to its short gestation period, relatively short lifespan, mild temperament, and the ease of obtaining animals with diverse health and genetic characteristics [18]. The current investigation utilizes rats as a model animal because recent research has reported that rats have been involved in a significant portion of the animal-based research on fractures in prominent orthopaedics over the past decade [19, 20].
4.2. Histological and Histomorphometric Analysis
Overall histology results revealed that the control and experimental groups had good healing pathways in all histological sections; however, there were variations in bone remodelling and deposition rates over the various periods.
4.2.1. Inflammatory Reaction
The present study illustrates an inflammatory reaction in study groups and different bone healing periods (7,14 days). In the experiments, rats were sacrificed on days 7 and 14 to evaluate different stages of wound healing and assess the effects of the treatments. On day 7, the early stages of wound healing were examined, including the presence of immune cells and cells involved in tissue repair. On day 14, the later stages of wound healing were observed, focusing on the formation of new tissue and the closure of the wound. By sacrificing the rats on these specific days, researchers gained important insights into the effects of the treatments at different time points, allowing for a comprehensive assessment of their efficacy throughout the wound healing process [21-23] .
It is now evident that inflammation is essential to healing bone defects. Through several hypothesized processes, such as neutrophilia or altered neutrophil priming and extension of osteoclast life along with lower osteoblast lifespans, increased inflammation may delay bone regeneration [24].
This is also consistent with the findings of [25], who demonstrated through their rat study that M. communis plays the largest role in the healing process of wounds. This plant increased the epithelialization process and reduced neutrophil numbers while increasing fibroblast cells.
4.2.2. Bone Cells
The current study shows an early stage of osteoid development. At seven days of healing, the mean difference in cell number between the experimental and control groups increased significantly, indicating that the M group is the most active in speeding the healing process, particularly in osteoblast mean count.
Results obtained with Myrtus communis disagree with those of [26], who tested the cytogenetic effect of Myrtus communis on bone marrow cells by infusion of 1 ml from oil and waiting for a period. The result showed that the M. communis infusions had statistically significant temporary inhibition of cellular division in the more concentrated M. communis infusion (2.5 mg/mL) and no alteration in the activity of bone marrow cells with time.
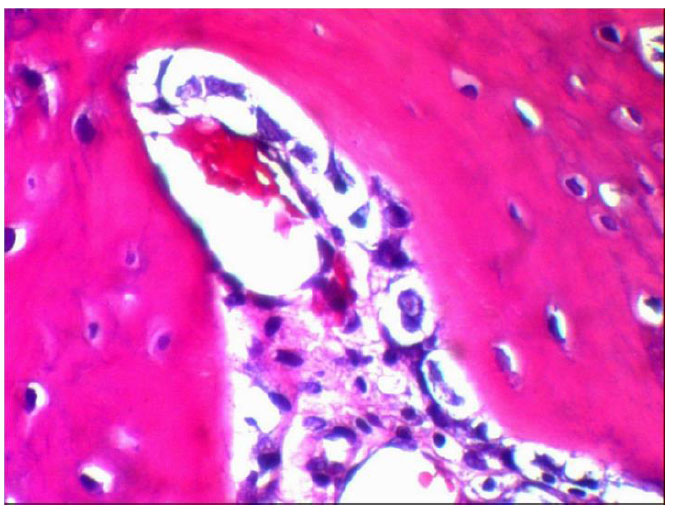
The formation of bone trabeculae in newly formed bone, as evidenced by histological sections that increased in size and quantity over the course of the 14-day intervals, and the development of bone healing, as shown by the deposition of matrix by differentiating osteoblasts over the course of the first week. The osteoclasts seen in the Howship lacunae are a sign of the remodelling process.
The results also showed a significant difference in the parameters related to the bone architecture of the M group. This could be explained by the fact that more formative cells, supplements, and nourishment are required for any new tissue formation, which would explain a gradual decrease in the number of osteoblasts. Furthermore, other than the maintenance of biological activity, no further osteoblasts or blood arteries were required after the bone production stabilized and attained its ultimate measurement.
CONCLUSION
Myrtus communis is an herbal material that has an anti-inflammatory effect, which is proven to decrease inflammatory cells and has a high effect on bone healing and regeneration in early bone matrix formation and minerali- zation, more than the normal physiological process.
Histological evaluation exhibits that myrtle oil motivates many osteoblasts osteocytes more than other groups, which our study proved has the highest effect on the acceleration of bone formation.
Histomorphometric results between groups showed a difference in the mean value of all bone architectural parameters, including bone cells (osteoblast, osteocyte, and osteoclast).
The present study showed that Myrtus communis effectively promoted osteogenesis, which may encourage future clinical surgical utilization.
RATIONALE
This study's findings could be useful in searching for novel, all-natural bone-healing remedies and compounds that can combat various dental conditions. Especially seek active medicines that don't interfere with the mouth's natural microbiome.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The investigation employing experimental study complied with the permission from the Kufa University/Najaf - IRAQ Local Ethical Committee (Reference number 5718).
HUMAN AND ANIMAL RIGHTS
All the animal experiments were performed according to the guidelines provided by the National Research Council. (All methods are reported in accordance with ARRIVE guidelines).
AVAILABILITY OF DATA AND MATERIALS
The data generated and analyzed in the present investigation can be provided upon a reasonable request.


