All published articles of this journal are available on ScienceDirect.
Herbal Medicine as an Adjunct in the Treatment of Periodontal Diseases - A Systematic Review
Abstract
Background
In recent times, a shift has been observed among both researchers and dental patients towards opting for herbal remedies and products to address issues like dental caries, gingivitis, periodontitis, periimplantitis, and dentin hypersensitivity. These herbal solutions encompass herbs, herbal constituents, formulations, and products derived from genuine plant components or other plant-derived materials.
Objective
This study aims to provide a comprehensive overview of the current body of literature regarding the added advantages of herbal medicinal products in managing periodontal and peri-implant conditions.
Methods
RCT published in English from 2013 till Sep 2023 in the terms “medicinal plants,” “plant medicine,” “dental herbs,” “medical herbs,” and “phytomedicine” were utilized to identify pertinent research papers. These search phrases were applied across the databases of PubMed, Scopus, and Web of Science. Case reports, case series, longitudinal studies, and retrospective analyses were not included. PRISMA standards were followed in this review.
Results
A total of fifteen (n=24) randomized clinical trials about herbal drugs and periodontal implications and three (n=3) trials on dental hypersensitivity were assessed. The trials assessed the use of various herbal products in treating periodontal and per-implant conditions.
Conclusion
Plant-based phytochemicals have anti-inflammatory effects that could be used as an alternative to treat periodontal and peri-implant conditions. Recent research on the different active components included in this study show significant improvements in the clinical parameters. To suggest them as substitutes for the non-herbal elements, more research is necessary.
1. INTRODUCTION
Untreated periodontitis, an ongoing inflammation of the gingiva and supporting structures of the teeth, can result in tooth loss [1-3]. It affects approximately 20-50% of the global population and 47.2% of Americans over 30 years old [4]. In 2019, Western Sub-Saharan Africa exhibited a high prevalence of standardized periodontal diseases, with the highest burden observed in the Gambia [5, 6]. An epidemiological investigation revealed that chronic periodontitis had the highest prevalence among the elderly population at 82%, followed by adults at 73% and adolescents at 59% [7]. The primary factor behind periodontitis is the presence of dental plaque-associated biofilm. Dental plaque consists of microbial biofilms adhering to the tooth surface [8]. The susceptibility of an individual to developing periodontitis is determined by their immune and inflammatory reactions to the bacteria within dental plaque, which can be influenced by environmental factors [9-11]. Risk factors for periodontitis encompass smoking, stress, poor dental hygiene, and genetic predisposition [12, 13]. Recent research underscores a significant link between periodontitis and conditions such as diabetes mellitus, cardiovascular disease, preterm birth, and lung diseases, highlighting the intricate relationship between oral health and overall well-being [14-16].
Gingivitis, characterized by inflammation of the marginal gingiva in response to tooth plaque, represents the most prevalent form of reversible periodontal disease and serves as the initial stage where periodontal disease becomes evident. Gingivitis can develop into periodontitis, which is permanent damage to the supporting connective tissue and alveolar bone when dental plaque is allowed to build up [17]. Thus, oral hygiene techniques that successfully eliminate/control plaque biofilm can inhibit and reverse gingivitis and periodontitis [18, 19]. The maintenance of regular dental hygiene, scaling, root debridement, antibacterial therapy, and surgical treatments are all part of the treatment for periodontal disorders [20-22].
The most advised and efficient ways to maintain oral hygiene and periodontal health are mechanical plaque management using a toothbrush and floss [23]. Toothpaste and mouthwashes with antimicrobial ingredients help control plaque by preventing the buildup of plaque biofilm, especially in hard-to-reach areas of the mouth [24]. The main method of preventing periodontal diseases is through the usage of dental care products like mouthwash and toothpaste. Chemical plaque control treatments can maintain oral hygiene in between brushings because of their strong oral substantivity. The effectiveness of various antimicrobial ingredients or agents in mouthwashes and toothpaste in reducing plaque and preventing periodontitis and gingivitis is debatable. Additionally, prolonged use of antimicrobial and anti-plaque mouth rinses is contra- indicated due to the possible associated side effects and the potential for the development of antimicrobial resistance. Therefore, alternative adjunctive approaches are being explored with minimal side effects. Across the world, both patients and clinicians are exploring minimally invasive periodontal treatments with minimal side effects. One such age-old method that has recently gained renewed interest is the use of herbal medicines to treat dental caries, gingivitis, periodontitis, periimplantitis, and dentinal hypersensitivity.
Herbal remedies include herbs, herbal components, preparations, and products made from actual plant parts or other plant substitutes. Medicinal plants contain various essential phytochemical compounds, including tannins, alkaloids, saponins, cardiac glycosides, steroids, terpenoids, flavonoids, phlobatannins, anthraquinones, and reducing sugars. To address inflammatory periodontal diseases, phytochemicals could be used as alternatives. They are phytoactive elements in plants renowned for their wound-healing, antimicrobial, and antibacterial qualities. [25, 26].
The increasing public interest in alternative medicine has spurred dental professionals to delve into established scientific research within this field. Several randomized clinical trials have been undertaken to evaluate the benefits of these alternative medicines. However, a comprehensive systematic review covering all the alternative medicines used in the treatment of periodontitis is currently lacking. Therefore, this article aims to systematically review the existing literature on the use of herbal medicines. The authors have chosen to focus their efforts on herbal therapy, also referred to as phytotherapy or phytomedicine, given the wide range of alternative medicine treatments available. Consequently, the primary objective of this study isto examine recent advancements in the effectiveness of various common compounds found in toothpaste and mouthwash products in addressing issues related to plaque, gingivitis, and periodontitis by analyzing studies published between 2013 and 2023.
2. METHODOLOGY
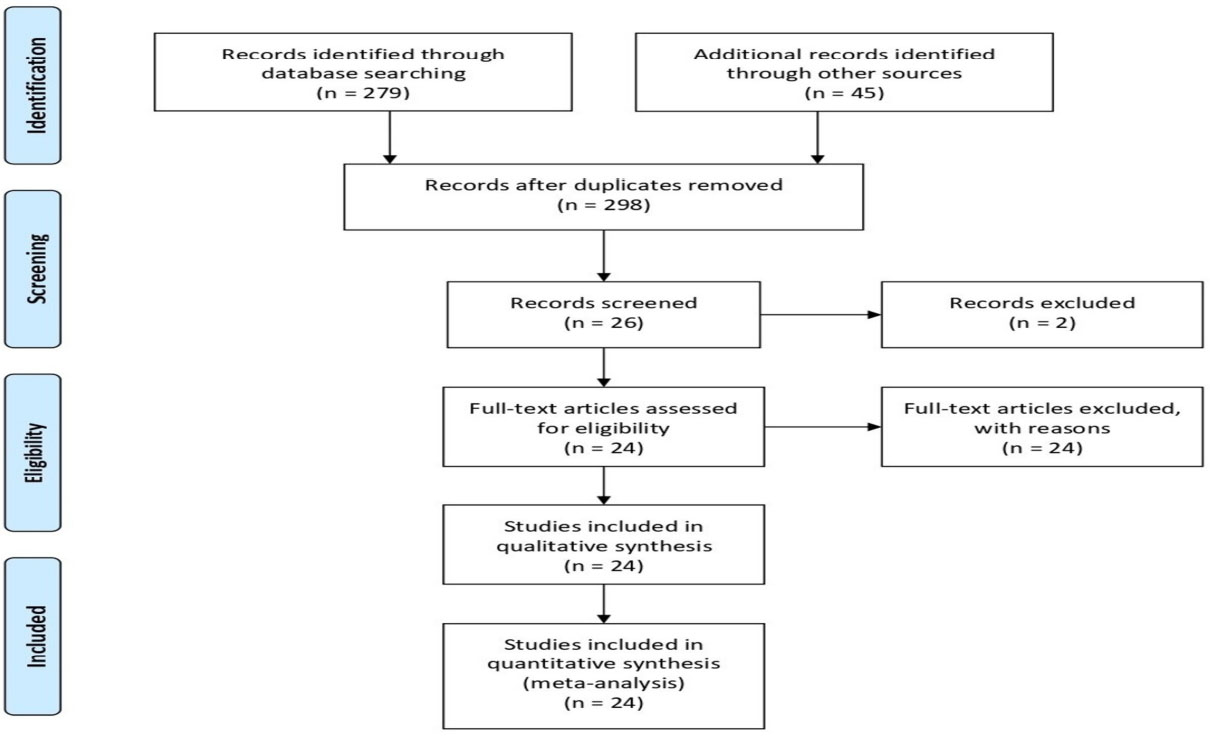
This review adhered to the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines. The research questions presented here align with the overarching objective of this systematic review (Fig. 1).
The three research questions were:
1. What is the latest research on utilizing therapeutic herbs for the treatment of periodontal disease?
2. What is presently understood regarding the utilization of medicinal herbs in peri-implantitis treatment?
3. What is the current understanding of medicinal herbs in addressing dentinal hypersensitivity?
Randomized Clinical Trials (RCTs) about the utilization of medicinal herbs in the treatment of gingival disease, periodontal diseases, peri-implantitis conditions, and hypersensitivity were considered for inclusion in this review. Case reports, case series, longitudinal studies, and retrospective analyses were excluded. Research articles that were not written in English were excluded. During the initial database search, various MeSH terms like “medicinal plants,” “plant medicine,” “dental herbs,” “medical herbs,” and “phytomedicine” were employed to identify relevant research papers. PubMed, Scopus, and Web of Science were the databases utilized to search for the relevant literature using the combination of MeSH words (medicinal plants + periodontitis; plant medicine + periodontitis; dental herbs + periodontitis; phytomedicine + periodontitis. These search terms were utilized in PubMed, Scopus, and Web of Science.
The process of collecting data and the specific data elements was conducted by two authors and recorded in a Microsoft Excel spreadsheet.
According to the PICOS question, a study needs to meet the following inclusion criteria.
(i) Healthy participants with gingivitis, periodontitis, or periimplantitis were selected.
(ii) The intervention group(s) should use a mouthwash containing plant extracts or essential oils.
(iii) The comparison group(s) should either receive a negative placebo solution or a mouthwash containing chlorhexidine (CHX).
(iv) All interventions should be additional to the participants' daily oral hygiene routines.
(v) The study should measure at least one plaque- or gingivitis-related index as an outcome measure.
(vi) Studies reporting specific bacteria outcomes.
(vii) Only Randomized Controlled Trials (RCTs) are preferred study designs as they are considered the gold standard for clinical research.
In vitro, studies, case reports, animal studies, long-term prospective studies, and research articles not written in English were excluded from the systematic review.
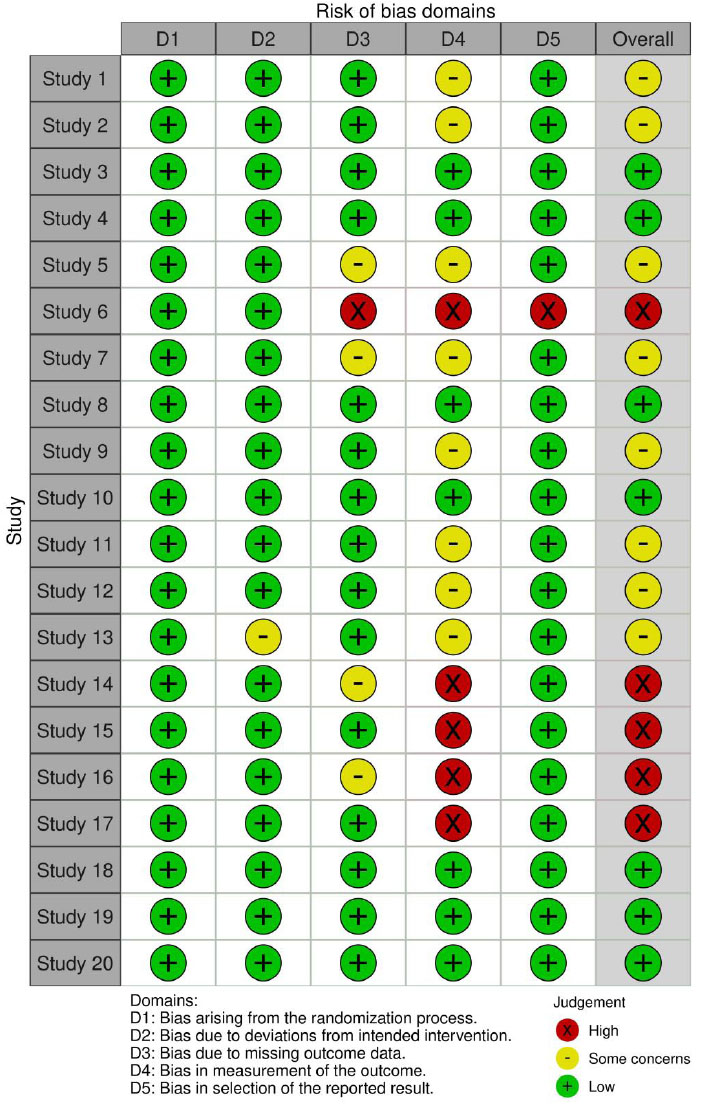
The evaluation of bias risk was carried out using Robvis, a web application developed during the Evidence Synthesis Hackathon. This online tool is based on the Robvis R package, which can be found at the provided link. The risk of bias table systematically assesses the methodological quality and potential biases in each included study. Researchers performing a meta-analysis or data synthesis will find the information in this table to be extremely helpful in determining the validity of the evidence and informing their conclusions regarding the overall caliber of the studies. Initially, all the included studies were independently assessed by the two authors and then reviewed in duplicate. In cases of disagreements, a third reviewer intervened to resolve any discrepancies. The risk of bias assessment was performed both within individual studies and across the studies. We evaluated the potential bias in each of the studies considered in our review. Our quality assessment was based on the guidelines employed by the (Risk of Bias) RoB 2.0 tool developed by McGuinness et al. in 2020 [25-27].
This assessment encompassed various domains relevant to our systematic review. We diligently reviewed each paper, considering the abstract, methodology, objectives, and results. Only publications that satisfied the criteria for high quality and met the eligibility standards were included in our current systematic review (Fig. 2).
The study used the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework by Guyatt et al. 2011 [28]. Initially, Randomized Clinical Trials (RCTs) were of 'high quality.' However, they were downgraded up to three levels to 'moderate quality,' 'low quality,' or 'very low quality' based on five specific factors: (i) risk of bias, (ii) indirectness of evidence, (iii) inconsistency of results, (iv) imprecision, and (v) publication bias. One primary reviewer conducted the assessments, which a second reviewer then reviewed. Disagreements were resolved through discussions with two additional reviewers.
3. RESULTS
An initial keyword search yielded 324 articles published between 2013 and October 2023. These articles were drawn from PubMed (195), Scopus (77), and Web of Science (52). Of these, 42 articles related to periodontal research underwent initial screening for relevance. Following a thorough review that aligned with the systematic review's goals, only 24 articles met the eligibility criteria. Among the eligible articles, 20 were sourced from PubMed, 2 from Scopus, and 2 from Web of Science. These articles specifically dealt with the use of medicinal plants in preventing gingival, periodontal, and peri-implant diseases.
The findings of the review suggest that herbal medicine could serve as a viable alternative to modern pharmaceuticals when used alongside scaling and root debridement. The alkaline properties of medicinal plants may play a role in their antibacterial effectiveness, helping to deter the development of plaque and calculus by maintaining a harmonious acid-alkaline balance in saliva. Importantly, it should be noted that while many periodontal pathogens display resistance to antibiotics, they are not impervious to the antibacterial properties of herbal medicine.
Furthermore, gingival tissues generally tolerate medicinal herbs quite well. In certain instances, the stems and leaves of different medicinal plants are used as natural toothbrushes and mouthwashes, offering a cost-effective way for dental patients to uphold oral hygiene, especially in developing nations. Additionally, formulations derived from plant extracts have the potential to both inhibit and treat periodontal conditions. The findings imply that miswak results in a significant reduction of PI and GI. Bamboo salt effectively reduced Streptococcus mutans and Lactobacillus species compared to non-herbal toothpaste (Table 1).
| S.No | Author/Year | Sample Size | Study Duration | Application Method | Active ingredient | Parameters Evaluated | Intervention / Control Group | Results | Standard of Care | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tadikonda A et al. 2017 [34] | n=46 | 30 days |
Dentifrice | Papain, bromelain, neem, and miswak with 1000 ppm fluoride (commercially available as Glodent vs Colgate strong teeth. | PI, GI | No | Results showed a significant reduction in PI and GI for Golden than Colgate strong | Non-surgical periodontal therapy, along with mouthwashes | The study was single-blinded & and plaque assessment in orthodontic patients was difficult. |
| 2 | Biria M et al. 2022 [35] | n=60 | 4 weeks | Dentifrice | Bamboo salt vs conventional non-herbal toothpaste | Streptococcus mutans and Lactobacillus species | No | Bamboo salt is potentially qualified as a complementary agent for self-care oral hygiene procedures. | Non-surgical periodontal therapy along with mouth washes | Blinding of participants and participants were dental students. |
| 3 | Mahyari S et al 2016 [36] | n=60 | 14 days |
Mouth wash | Polyherbal mouth wash (Z. officinale, R. officinalis, C. officinalis) vs CHX vs placebo | Gingival and plaque indices | Yes | Polyherbal mouthwash showed similar results to CHX | Non-surgical periodontal therapy along with mouth washes | Short follow up |
| 4 | Pradeep et al 2016 [37] | n=90 | 60 days |
Mouth wash | Triphala vs CHX vs placebo | PI, GI, OHI-S, and microbiologic counts | Yes | TRP mouthwash decreased all parameters and was comparable to CHX | Non-surgical periodontal therapy along with mouth washes | The Hawthorne effect was reported |
| 5 | Vangipuram S et al 2016 [38] | n=390 | 30days | Mouth wash | Aloe Vera (AV) vs chlorhexidine group (0.12% CHX) | GI, PI | Yes | AloeVera has shown equal effectiveness as CHX. | Non-surgical periodontal therapy along with mouth washes | The sample size includes undergraduates, postgraduates, and dental interns |
| 6 | Chhina S et al 2016 [39] | n=90 | 4days | Mouth wash | Aloe vera vs 0.2% CHX, vs Placebo group | Quigley Hein Modified Plaque Scores | Yes | AV mouthwash has comparable antiplaque efficacy as the gold standard 0.2% CHX | Non-surgical periodontal therapy along with mouth washes | A 4-day plaque regrowth model is applied, which is insufficient to assess gingival scores. |
| 7 | Chatterjee A et al 2017 [40] | n=150 | 28days | Mouth wash | 0.1% curcumin vs 0.2% CHX vs placebo | Plaque, gingival, and sulcus bleeding indices | Yes | Curcumin mouthwash has shown antiplaque and anti-gingivitis properties comparable to CHX mouthwash. | Non-surgical periodontal therapy along with mouth washes | Short follow up period |
| 8 | Irfan M et al. 2018 [41] | n=50 | 45 days | Mouth wash | Triphala vs 0.2% CHX | GI, PI | No | Triphala mouthwash has similar results in comparison with CHX in reducing GI, PI in CP patients. | Non-surgical periodontal therapy along with mouth washes | No placebo group in the study design |
| 9 | Nayak et al 2019 [42] | n=60 | 90 days | Mouth wash | Guava leaf extract, vs 0.2% CHX, placebo | GI, PI and microbial counts | Yes | Guava leaf extract mouth rinse showed better results than placebo but inferior to CHX. | Non-surgical periodontal therapy along with mouth washes | No specific bacteria were evaluated instead CFU levels were evaluated |
| 10 | Sparabombe S et al 2019 [43] | n=34 | 90 days | Mouth wash | Polyherbal mouthwash with Propolis resin extract, Plantago lanceolata, Salvia officinalis leaves extract vs. 1.75% essential oils | Full mouth bleeding score (FMBS), full mouth plaque score (FMPS), PD, CAL | Yes | Polyherbal mouthwash has a great effect in decreasing BOP scores and plaque accumulation. | Non-surgical periodontal therapy along with mouth washes | The study design has only a placebo as a control group and did not have CHX as a positive control group |
| 11 | Penmetsa G et al. 2019 [44] | n=-60 | 1 month | Mouth wash | Triphala vs. Aloe vera vs. CHX mouthwash | PI, GI, bleeding index (BI) | No | Triphala effectively reduced PI, GI, and BI in comparison to aloe vera and was comparable to CHX | Non-surgical periodontal therapy along with mouth washes | Small sample size |
| 12 | Malekzadeh M et al 2020 [45] | n=48 | 28 days | Mouth wash | Sina Curcumin capsules 80mg vs placebo. | Papillary bleeding index (PBI), and modified gingival index (MGI) | Yes | The results showed a decrease in inflammation and gingival bleeding in patients when using Curcumin capsules. Also, no side effects were noticed. | Non-surgical periodontal therapy, along with mouth rinsing | Evaluation of solely the clinical outcomes |
| 13 | Andhare MG et al 2022 [46] | n =60 | 21 days | Mouth wash | 0.5% green tea vs 0.2% CHX vs aloe vera mouthwash | PI, GI, sulcular bleeding index (SBI) | Yes | Green tea mouthwash displayed a significant reduction in PI, GI, SBI. | Non-surgical periodontal therapy along with mouth rinses | Small sample size and short-term follow-up |
| 14 | Kim YR et al. 2022 [47] | n=64 | 5 days | Mouth wash | Control group saline gargle vs. Sambucus williamsii var. coreana extract | PI, GI, Microbiological assessment | No | Significant reduction in PI and GI with mouth wash. | Non-surgical periodontal therapy | Small sample size and short-term follow-up |
| 15 | Singh A et al. 2018 [48] | n=120 sites | 3 months | Chip | SRP+CHX chip vs SRP+turmeric chip vs SRP alone | PI, GI, PPD, and relative attachment level (RAL) | Yes | Both LDD agents as an adjunct to SRP proved to be equally beneficial in the treatment of CP. | Non-surgical periodontal therapy along with LDD | Small sample size |
| 16 | Kaur H et al. 2019 [49] | n=30 | 12 weeks | Gel | SRP vs SRP with subgingival application of curcumin gel | Clinical parameters and Saliva analysis for IL-1β was done by ELISA. | Yes | Single application of curcumin (turmeric) gel has limited added benefit over SRP in CP patients. | Non-surgical periodontal therapy along with LDD | Small sample size |
| 17 | Siddharth M et al.2020 [50] | n=25 | 3 months | Gel | 2% curcumin gel vs 0.2% CHX | Sulcus bleeding index (SBI), PPD, and relative attachment level (RAL) and CFUs | Yes | 2% curcumin gel revealed notable improvement in sulcus BOP, PPD, CAL when delivered to pockets in comparison with 0.2% CHX gel. | Non-surgical periodontal therapy, along with mouth washes | Small sample size. No placebo effect was evaluated. |
| 18 | Pérez-Pacheco, C.G et al 2020 [51] | n=20 | 180 days | Nonoparticles | SRP+PLGA/PLA nanoparticles loaded with 50 μg of curcumin (N-Curc) vs SRP+empty nanoparticles. | PPD, CAL, and BOP. IL-1α, 6, 10 and TNFα, in GCF assessed by ELISA. 40 bacteria determined by DNA hybridization. | Yes | Single local administration of nanoencapsulated curcumin in PD sites had no additive benefits to Non-surgical periodontal therapy. | Non-surgical periodontal therapy | Small sample size |
| 19 | Alzoman H et al 2020 [52] | n=48 | 12 weeks | Mouth wash | Herbal-based oral rinses vs 0.12% CHX | Peri-implant plaque index (PI), BOP, PD | Yes | Herbal and 0.12% CHX-based mouthwash are useful adjuncts to MD for the treatment of peri-implant mucositis. | Non-surgical mechanical debridement followed by mouthwashes. | Small sample size and short-term follow-up |
| 20 | Alqutub MN et al 2023 [53] | n=60 | 12 weeks | Mouthwash | 0.12% CHX-group vs. Sodium chloride (NaCl) group vs 2% NaCl rinses vs Herbal MW group | Peri-implant modified plaque index (mPI), modified gingival index (mGI) and probing depth (PD) | Yes | After mechanical debridement, post-operative use of CHX and herbal and NaCl mouthwash is useful for the management of PiM in the short term. | Non-surgical mechanical debridement followed by mouth rinses | Small sample size and short-term follow-up |
| 22 | Kumari M et al 2013 [54] | n=73 | 12 weeks | Dentifrice | Placebo dentifrice vs Hi Ora K | Sensitivity scores for controlled air stimulus and cold water were recorded. | Yes | Hi Oral K reduces sensitivity significantly compared to placebo. | Application of desensitizing agents | No positive control group |
| 23 | Kumari M et al 2016 [55] | n =145 | 12 weeks | Dentifrice | Placebo vs Spinacia oleracea herbal, vs 5% potassium nitrate. | Hypersensitivity scale values were assessed by tactile, cold, and air tests | Yes | Herbal dentifrice displayed similar results to the non-herbal dentifrice, and it can be recommended for the treatment of DH. | Use of desensitizing agents | Lack of standardization in dentifrices formulations |
| 24 | AlQahtani SM et al 2023 [56] | n=75 | 28 days | Gel | 10% propolis hydrogel vs 2% NaF vs 1.23% APF in conjunction with iontophoresis | Hypersensitivity scale values were assessed by tactile, cold, and air tests | Yes | 10% propolis hydrogel can be used as a naturally occurring alternative to commercially available fluoridated desensitizers | Application of desensitizing agents | Minimum follow-up time. |
Z. officinale, R. officinalis, C. officinalis, Glycyrrhiza glabra, Triphala, Aloe Vera (AV), curcumin, guava leaf extract, green tea, and Sambucus williamsii var. coreana extract demonstrated significant efficacy in reducing Plaque Index (PI), Gingival Index (GI), Bleeding on Probing (BOP), and addressing halitosis when utilized as mouthwashes. Particularly, curcumin, when employed in various forms as a Locally Delivered Drug (LDD) agent, outperformed the control group, showing promise as an alternative for managing periodontitis conditions. Further more, herbal mouthwashes proved effective in diminishing peri-implant PI, Pocket Depth (PD), and BOP compared to Chlorhexidine (CHX), suggesting their potential use as alternatives to conventional mouthwashes. Hi Ora K and Spinacia oleracea herbal tooth were effective in reducing tooth hypersensitivity compared to regular desensitizing toothpaste, as demonstrated in Table 1.
4. DISCUSSION
The primary findings of this systematic review underscore the significance of utilizing diverse herbal remedies for addressing gingival issues, periodontal problems, peri-implantitis, and dentinal hypersensitivity. Furthermore, current studies affirm the customary application of medicinal herbs in managing peri-implantitis, periodontal conditions, and gingival disorders [29, 30]. The effectiveness of medicinal plants in reducing periodontal and peri-implantitis diseases can be ascribed to their anti-inflammatory, antioxidant, antibacterial, astrin gent, and other therapeutic properties. It's important to note that only Randomized Controlled Trials (RCTs) were considered due to their ability to provide the most reliable evidence regarding intervention effective- ness. RCTs are preferred because their methodology minimizes the influence of confounding factors on the results.
One notable advantage of using medicinal herbs as opposed to chemotherapeutic medications is the reduced likelihood of untoward responses, including hyper sensitivity and the emergence of bacterial resistance often linked to conventional medications. For instance, CHX evokes adverse effects such as allergic contact dermatitis and anaphylaxis [31, 33]. The collective therapeutic attributes of medicinal plants render them beneficial in alleviating gingival and periodontal conditions, which constitute one of the secondary outcome measures of using medicinal herbs.
In a 2017 paper by Tadikonda A. et al., the impact of using miswak dentifrice was examined concerning plaque index (PI) and gingival index (GI) compared to Colgate Strong toothpaste. The study found a notable decrease in both PI and GI, signifying its effectiveness in reducing these parameters by this herbal toothpaste. Additionally, in 2022, research conducted by Biria M. et al. evaluated the effects of Bamboo salt on colony-forming units of S. mutans and Lactobacillus species. The findings indicated that Bamboo salt has the potential to serve as a valuable complementary agent for self-care oral hygiene practices [34, 35] (Table 1).
Studies conducted by Mahyari S et al. in 2016, Pradeep et al. in 2016, Vangipuram S et al. in 2016, Chhina S et al. in 2016, Chatterjee A et al. in 2017, Irfan M et al. in 2018, Nayak et al. in 2019, Sparabombe S et al. in 2019, Gouthami et al. in 2019, Malekzadeh M et al. in 2020, Mangesh G et al. in 2022, and Kim YR et al. in 2022, individually investigated the effects of various herbal types of mouthwash, including Aloe vera, Curcumin, green tea, Guava leaf extract, Triphala, and Sambucus williamsii var. coreana extract mouthwashes. The findings from these studies demonstrated the efficacy of these herbal mouthwashes in reducing PI, GI, and BOP, while significantly enhancing overall gingival and periodontal health. Notably, these improvements were achieved without any reported side effects, as compared to the use of CHX [36-47] (Table 1).
Research carried out by Singh A. et al. in 2018, Kaur H et al. in 2019, Siddharth M et al. in 2020, and Pérez-Pacheco, C.G et al. in 2021 evaluated clinical parameters like PI, GI, and PPD in comparison to CHX or scaling and root debridement. Their findings indicated significant enhancements in measurements such as BOP, PPD, clinical attachment level (CAL), and a reduction in inflammatory mediators when delivered into pockets, as opposed to using 0.2% CHX gel [48-51] (Table 1).
Studies conducted by Alzoman H et al. in 2020 and Alqutub MN et al. in 2023 investigated the impacts of herbal medications on peri-implantitis tissues, focusing on parameters such as PI), probing depth (PD), and bleeding on probing (BOP). Their findings revealed a notable decrease in all these indices and suggested that the use of herbal mouthwash is a valuable short-term strategy for managing peri-implantitis [52, 53]. Table 1. Kumari M et al. 2013, Kumari M et al. 2016 and AlQahtani SM et al. 2023 used natural herbal agents to treat dental hypersen-sitivity [54-56] (Table 1).
The current article provides an overview of the diverse therapeutic benefits of medicinal herbs in the context of oral health. This exploration serves to shed light on the importance of addressing periodontal and peri-implant diseases through herbal interventions. It's worth noting that two notable limitations of the study include the scarcity of evidence-based research regarding the use of herbal remedies and the limited number of randomized clinical trials available to assess the effectiveness and safety of traditional herbal treatments. Other limitations include variations in sample size and follow-up periods among different studies. At the out level, the reduction in gingival and periodontal conditions varied among different studies, leading to variability in the reported effectiveness of herbal mouthwashes compared to CHX. Other drawbacks include limited data on the adverse effects of herbal products in terms of safety in comparison to CHX. In addition to this, we lack evidence of the long-term impact of these herbal products compared to CHX. Lastly, a lack of standardization of herbal products makes it challenging to compare different products and assess their relative efficacy and safety accurately. The use of lysates and postbiotics can modify clinical and microbiological parameters in periodontal patients, so these products should also be considered in future trials, as adjuvants and in combination with herbal remedies [57]. The heterogeneity of the studies, the criteria used for inclusion and exclusion of the studies, and the different primary and secondary outcomes used for the studies make undertaking a meta-analysis difficult. Hence, only a systematic review was undertaken, and no meta-analysis of the data was performed. Studies related to dentinal hypersensitivity are mentioned in the table below.
The studies have been conducted on varied populations, including gingivitis, periodontitis, mild periodontitis, and periodontal health. The standard of care in patients with periodontal health is reinforcement of oral hygiene instructions. The standard of care in gingivitis patients is oral hygiene instructions and oral prophylaxis. The standard of care in periodontitis patients is scaling and root debridement. Most of the studies have provided a standard of care to all the patient groups in the form of oral hygiene instructions, oral prophylaxis, and Scaling and root debridement. For a better understanding of the reviewer, a column has been added regarding the standard of care in Table 1.
The majority of the studies are in the trial stage and evaluation stage. The products to be approved by the FDA and ADA would require an enormous amount of time and funding for approval by these regulatory authorities. The same has been mentioned in the limitations section of the review. The majority of the included articles are randomized controlled trials or longitudinal studies and are published in Scopus-indexed and PubMed-indexed articles. So, it would be considered that these studies have IRB approval.
CONCLUSION
Plant extract formulations, with their analgesic, anti-inflammatory, antibacterial, and antioxidant properties, have the potential to both prevent and treat gingival, periodontal, and peri-implant diseases. They can serve as a practical substitution for current pharmaceuticals when combined with scaling and root debridement procedures. Nevertheless, it's essential to emphasize that additional clinical trials are required to validate the effectiveness of herbal medicine as a therapeutic approach for periodontal treatments.


