All published articles of this journal are available on ScienceDirect.
An Analogy between Two Bio-Membranes (CGF-PRGF) Mixed with Xenogeneic Bone Graft to Achieve Frugal Management of Labial Dehiscence amidst Immediate Implantation in Esthetic Zone: A Randomized Clinical Trial
Abstract
Background
Labial dehiscence is a very prevalent esthetic concern among implantologists during immediate implantation in the esthetic zone since recent implant treatment and patient ambition are not only directed to function reestablishment, but esthetic superbness also holds immense consideration.
Objective
This study aimed to manage the labial dehiscence at the time of implantation and evaluate and compare the effects of two different autologous biomembranes, Concentrated Growth Factor (CGF) and Plasma Rich Growth Factors (PRGF), in combination with xenogeneic bone around the immediate implants in the esthetic zone.
Methods
Twenty patients indicated for immediate implant in the esthetic zone were randomly divided into two groups. The first group received CGF with xenograft, and the second group received PRGF mixed with xenograft. Cone Beam Computed Tomography (CBCT) radiographs and clinical periodontal parameters were evaluated, and all the results were tabulated and statistically analyzed.
Results
The two groups showed a statistically significant increase in bone density and inevitable crestal bone loss after 9 months, even though the CGF group comparably showed a statistically significant reduction in crestal bone loss.
Conclusion
Platelet concentrate derivatives, such as CGF and PRGF, are beneficial in the management of labial dehiscence around the immediate implants even though CGF offers a considerably and statistically significant decrease in crestal bone loss and more amelioration of bone density alongside its easier and faster preparation than PRGF.
Clinical Trial Registration
The study is registered at the U.S. National Library of Medicine website of clinical trials (clinicaltrials.gov) under ID: NCT05595772.
1. INTRODUCTION
Immediate Implant Placement (IIP) offers tooth replacement instantaneously with a concurrent decrease in surgical maneuvers and increasing patient satisfaction, so it has been scientifically highlighted over the past two decades [1-3]. One of the major obstacles in IIP, which may jeopardize the final esthetic outcome, is labial plate dehiscence [4]. Dehiscence is a highly prevalent bone deformity approaching apically in a V-shaped manner with subsequent divesting of the root surface or implant fixture surface of its supporting tissues [5]. Several methods and maneuvers have been propounded to manage labial dehiscence around the immediate implants, some of which include taking advantage of platelet concentrates formulated by various blood extractors and activators. Using platelet concentrates yields autogenously acquired and highly concentrated growth factors, guaranteeing biocompatibility and superior wound healing [6-13]. Mixing these formulations with xenogeneic bone may grant higher implant success, i.e., up to 100%, besides enhanced structural unity and greater mechanical stability, avoiding complications of donor site in contrast to autogenous bone grafts and high cost in comparison to allogeneic grafts [14-18].
PRGF is an effortlessly obtained Autologous Platelet Concentrates (APCs), and it is a subclass of Pure Platelet-Rich Plasma (P-PRP) widely utilized clinically in dentistry and profusely in regenerative medicine due to its simple preparation technique and exorbitant concentration of growth factors, which facilitate tissue regeneration and motivate tissue healing [19-21].
It has been reported that PRGF exhibits a beneficial effect in all intraoral augmentation procedures in light of its healing propensity, but notably in vertical augmentation and management of dehiscence and reduction of post-surgery complaints [22-25].
Concentrated Growth Factor (CGF) is one of the newest APCs proposed by Sacco in 2006 and is considered a third generation of platelets products; its formulation step is simpler than PRGF, and it has more intricate and three-dimensional fibrin reticulation that stipulates a denser fibrin matrix and richer growth factors that liberate gradually, thus providing superior healing [26-30].
Anitua et al. investigated the use of PRGF thoroughly, and in their conclusion, they encouraged the utilization of PRGF in oral surgical procedures to improve the healing processes of the oral soft and hard tissues. Moreover, a systematic review by Gupta et al. stated that CGF could aid in gaining vertical bone around the implant when used with xenogeneic bone and improve the quality of newly formed bone [23, 31-33]. Hence, this study was designed to evaluate and compare the effect of economically prepared PRGF and CGF biomembranes with xenogeneic bone to manage labial dehiscence around the immediate implants using CBCT to measure marginal bone level and bone density around implants, which will significantly and frugally improve the durability and esthetics of implant restorations.
2. MATERIALS AND METHODS
This study was approved by the Institutional Ethics Committee and informed written consent was obtained from the participants prior to their participation.
2.1. Subject Population
2.1.1. Sample Size Calculation and Randomization
The required number of patients in each group was determined after a power calculation using G Power 3.1 9.2 software. Data obtained from previous literature were used to calculate the effect size [34]. Considering bone density as the main parameter, this yielded a minimum sample of 20 patients to provide 80% power at the level of 5% significance. So, the study was conducted on 20 patients indicated for immediate implant placement in the esthetic zone, and the patients were recruited from the outpatient Clinic of Oral Medicine, Oral Diagnosis, and Periodontology Department, Faculty of Dentistry, Minia University. Patients were enrolled by authors randomly using a flip coin into one of the two groups as follows:
Group A (received immediate implant + CGF and xenograft) = 10 patients
Group B (received immediate implant + PRGF and xenograft) = 10 patients
2.2. Inclusion Criteria
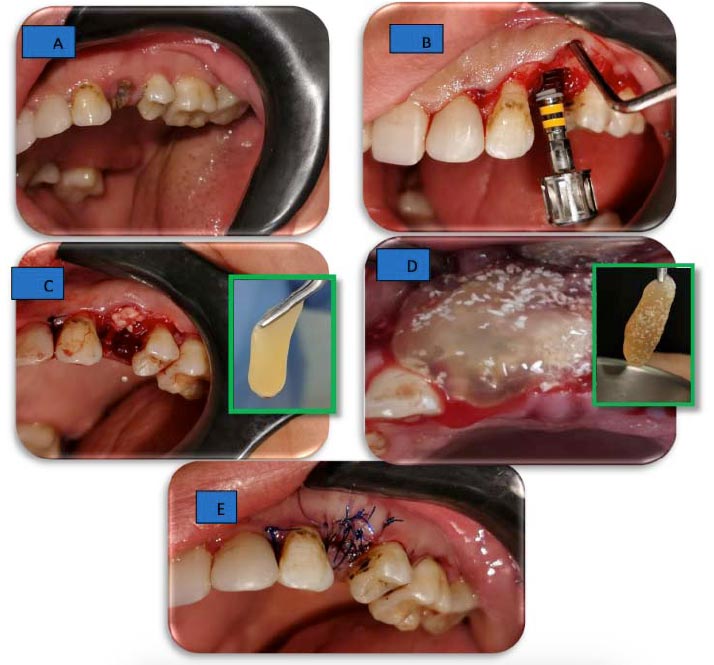
Selected patients of both sexes were 20-40 years old and systemically healthy based on dental modification of Cornell index questionnaire, classified as having gingival health according to 2017 classification of periodontal and peri-implant diseases and conditions, and adequate interocclusal space at least 8–12 mm in vertical distance, expected class II extraction socket according to Li-Chang 2021 classification of extraction sockets with adequate native apical bone, and being free from any acute pathological conditions (Fig. 1).
2.3. Exclusion Criteria
Pregnant women, smokers, and patients with parafunctional habits or periodontitis were excluded.
2.4. Surgical Phase
2.4.1. Flap Elevation and Implant Insertion
After the preparation of the surgical site using povidone-iodine 7.5% (Betadine 7.5%, the Nile Comp. for Pharmaceuticals and Chemical Industries, Alexandria, Egypt) and anesthetization using Articaine 4% with epinephrine 1:100,000 (Inibsa, Barcelona, Spain), the elevation of intrasulcular flap and atraumatic extraction of the tooth or remaining root were performed, followed by sequential and copiously irrigated implant drilling (IS-II active Neobiotech® Neobiotech Co., Ltd Seoul, Republic of Korea) and insertion according to ideal 3D position of the implant (Figs. 2A and B).
A sample of venous blood was withdrawn, and the membrane was prepared without delay according to the preparation protocol of each group:
2.4.2. Membrane Preparation Protocol
2.4.2.1. Group A (Protocol for CGF Preparation)
A total of 10 ml venous blood samples were withdrawn for centrifugation, which was carried out using a benchtop adjustable speed and time matching centrifuge device (CenTrKinG ET-12M Egyptian Trade Co. Cairo, Egypt) and centrifuge tubes without anticoagulants in an opposing balanced manner. The device was preprogrammed by a specialized technician to mimic the proposed method of preparation of CGF (accelerated for 30s, rotated in four sequential steps, and finally decelerated for 36s) (Table 1) [35-37].
| Step No. | Gravitational Force | Equivalent RPM | Duration |
|---|---|---|---|
| 1 | Acceleration from zero | Acceleration from zero | 30 seconds |
| 2 | 735 g | 2249 ≈ 2200 | 2 minutes |
| 3 | 580 g | 1998 ≈ 2000 | 4 minutes |
| 4 | 735 g | 2249 ≈ 2200 | 2 minutes |
| 5 | 905 g | 2495 ≈ 2500 | 3 minutes |
| 6 | Deceleration from 905g | Deceleration from 2500g | 36 seconds |
2.4.2.2. Group B (Protocol for PRGF Preparation)
A total of 30 ml venous blood was collected and deposited in 5 mL tubes containing sodium citrate anticoagulant, which were centrifuged at 580 G (2000 rpm) for 8 minutes at room temperature. After centrifugation, the blood sample was layered into four distinctive layers: 1) 0.5 mL Plasma Poor in Growth Factors (PPGFs) = F1 in the uppermost part of the tube; 2) 0.5 mL Plasma with Growth Factors (PGFs) = F2; 3) 0.5 mL Plasma Rich in Growth Factors (PRGF) = F3 located immediately above the red blood cell portion in the tube; 4) red blood cell concentrate layer. The PRGF (F3) was separated from all tubes using 500 μL pipettes and transported to an independent dish, and then activated using 50 μL of 10% calcium chloride for every 1 ml of preparation and mixed with xenogeneic bone graft. It was then incubated for 40 minutes at 37°C in a water bath (Water bath Analog acrylic BTC -Biotech Company for Medical and Laboratory Equipment. Cairo, Egypt) to produce an easy-to-handle gelatinous layer (PRGF) of fibrin loaded with xenogeneic bone [38, 39].
2.4.3. Bone Grafting and Membrane Insertion
The membranes were then applied (Figs. 2C and D) and condensed around the dental implant, either loaded with or covered with xenogeneic bone (OneXeno Graft® - OneGraft, Berlin, Germany) to fill the gap between the fixture and the walls of the socket to rebuild the area of dehiscence. Finally, tension-free closure of the flap was achieved using 4/0 propylene sutures (Fig. 2E).
2.5. Prosthetic Phase
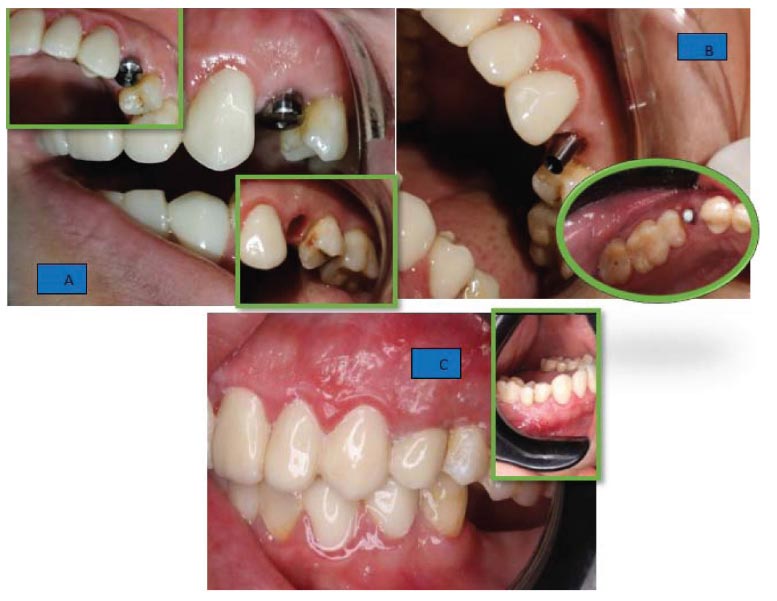
After a 3-month healing period, the second stage of implantation was performed by detaching the cover screw of the implant and attaching the healing abutment for 2 weeks; then, it was replaced with a suitable regular straight or angled abutment and temporary cemented zirconium crown (Fig. 3).
2.6. Radiographic Assessment
CBCT was performed at baseline and 9 months again after removal of the temporary cemented crown and unscrewing of the abutment. The cover screw was reattached to prevent artifacts in CBCT and obtain standardized measurements.
2.6.1. Crestal Bone Loss
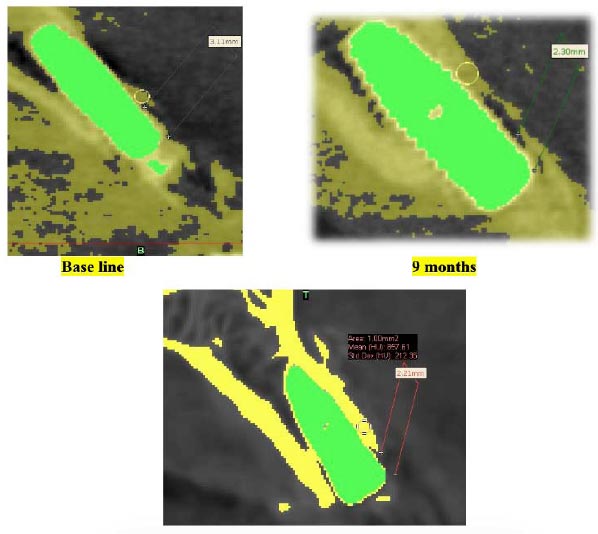
A standardized, reproducible vertical line in the center of the implant from the sagittal cut in the anterior teeth and coronal cut in the first premolars was used in coincidence with two horizontal tangential lines, one from the platform of the implant and the other from the level of the crestal bone. The distance between the two lines was considered the crestal bone level (Fig. 4A) [40, 41].
2.6.2. Bone Density
First, a single threshold value was selected based on a local gray level value and image gradient to create a mask to separate the target from the background. The masking was done according to the grayscale threshold referring to the grayscale of the different tooth structures and bone, and then the mask was drawn and erased manually layer by layer in at least two orientations to separate the implant from the bone. At last, we smoothed and adjusted the mask according to the target border using the “Contour Edit” in the software. After threshold separation, the bone density of each buccal surface was measured. After that, we calculated the density by Hounsfield unit area and standard deviation (Fig. 4B) [42-44].
2.7. Clinical Assessment
2.7.1. Keratinized Tissue Width (KTW) and Gingival Thickness (GT)
KTW and GT were evaluated at baseline and again at 3 months, 6 months, and 9 months. A standardized acrylic stent (Acrostone Acrylic Material - Cold Cure and Acrostone Co., Ltd. Cairo, Egypt) of 1mm thickness was made preoperatively for every patient to ensure accurate reproducibility of the sites of measurement (Table 2).
2.7.2. Probing Depth (PD), Modified Gingival Index (MGI), and Modified Plaque Index (MPI)
PD, MGI, and MPI were evaluated after loading of a temporary cemented crown (3 months), and at 6 and 9 months (Table 2).
| S.No. | Type of Assessment | Assessment | Timing of Assessment | ||||
|---|---|---|---|---|---|---|---|
| 1st Stage | Baseline | 3 Months | 6 Months | 9 Months | |||
| 2nd Stage | - | Baseline | 3 Months | 6 Months | |||
| 1 |
Radiographic assessments CBCT |
Crestal bone level | √ | - | - | √ | |
| 2 | Bone density | √ | - | - | √ | ||
| 3 | Clinical assessments | Keratinized Tissue Width (KTW) | √ | √ | √ | √ | |
| 4 | Gingival Thickness (GT) | √ | √ | √ | √ | ||
| 5 | Probing Depth (PD) | - | √ | √ | √ | ||
| 6 | Modified Gingival Index (MGI) | - | √ | √ | √ | ||
| 7 | Modified Plaque Index (MPI) | - | √ | √ | √ | ||
2.8. Statistical Analysis
Statistical analysis was performed using IBM® SPSS® software (ver. 26. SPSS Inc., IBM Corporation, Armonk, NY, USA). Data were explored for normality using the Shapiro-Wilk test. Quantitative data are presented by mean and standard deviation. An independent t-test was used to compare the mean between two independent groups, and a paired t-test was used to compare the pre- and postoperative mean within the same group. Qualitative data are expressed as numbers and percentages. The Chi-square/exact test was used to compare proportions. A statistically significant level was considered when the p-value was < 0.05 and highly statistically significant at < 0.001 or less.
3. RESULTS
Twenty patients were recruited in the study, including 16 females and 4 males aged between 20 to 40 years; there were no failed implants or withdrawal of any patients during the follow-up period. Data are expressed as mean ± standard deviation.
3.1. Radiographic Parameters
3.1.1. Crestal Bone
Both groups showed crestal bone loss postoperatively, and it was highly statistically significant in both groups. Comparing the baseline values of the CGF group vs. PRGF group, they were not statistically significant, while postoperative values showed a high statistical significance.
Notably, the mean difference and percentage of change were much less in the CGF group than in the PRGF.
3.1.2. Bone Density
Both groups showed increased bone density postoperatively, and it was statistically significant. The baseline values of the CGF group vs. the PRGF group were not statistically significant, while the comparison of postoperative values showed statistical significance.
The analyzed datasets of radiographic assessments are tabulated in Tables 3-5.
3.2. Clinical Parameters
3.2.1. Inter-group Result
3.2.1.1. CGF
KTW increased after 3 months, and it declined slightly at 6 months but was still more than that at baseline. Afterward, at 9 months, it showed an increase again. GT showed a constant and statistically significant increase throughout different follow-up periods. PD showed a highly statistically significant decrease throughout different follow-up periods. Both MGI and MPI were found to be improved throughout the follow-up period.
| Parameters | - | Group | P-value a | |
|---|---|---|---|---|
| CGF (n= 10) Mean±SD |
PRGF(n=10) Mean±SD |
|||
| Crestal bone level | At baseline postoperative |
2.68±0.52 1.97±0.44 |
2.23±0.77 1.16±0.47 |
0.148 0.001 ** |
| Bone density level | At baseline postoperative |
483.9±246.1 660.3±250.8 |
787.2±422.0 959.2±360.3 |
0.064 0.044 * |
| - | Groups |
Baseline Mean±SD |
Postoperative Mean±SD |
Mean difference | P-valuea |
|---|---|---|---|---|---|
| Crestal bone level | CGF | 2.68±0.52 | 1.97±0.44 | 0.418 | 0.001 ** |
| PRGF | 2.23±0.77 | 1.16±0.47 | 1.07 | 0.001 ** | |
| Bone density level | CGF | 483.9±246.1 | 660.3±250.8 | -176.4 | 0.024* |
| PRGF | 787.2±422.0 | 959.2±360.3 | -172.0 | 0.043* |
| - | Group | P-valuea | ||
|---|---|---|---|---|
| CGF (n= 10) Mean±SD |
PRGF (n=10) Mean±SD |
- | ||
| Bone loss | -0.26±0.14 | -0.46±0.24 | 0.036* | |
| Clinical Parameter | Group | P value | |||
|---|---|---|---|---|---|
| CGF (n= 10) Mean±SD |
PRGF (n=10) Mean±SD |
||||
| Keratinized tissue width | At baseline | 4.10±0.87 | 4.00±0.94 | 0.809 | |
| 3 months | 4.45±1.01 | 3.75±1.06 | 0.148 | ||
| 6 months | 4.25±0.97 | 3.40±1.37 | 0.128 | ||
| 9 months | 4.40±0.96 | 3.40±1.37 | 0.076 | ||
| Gingival thickness | At baseline | 3.20±0.53 | 3.45±0.55 | 0.318 | |
| 3 months | 3.60±0.56 | 3.42±0.46 | 0.914 | ||
| 6 months | 3.75±0.48 | 3.85±0.66 | 0.169 | ||
| 9 months | 3.95±0.59 | 3.75±0.56 | 0.461 | ||
| Probing depth | 3 months (2nd stage baseline) | 5.30±0.63 | 4.95±0.59 | 0.220 | |
| 6 months (2nd stage 3 months) | 3.35±0. 41 | 4.10±0.45 | 0.001** | ||
| 9 months (2nd stage 6 months) | 3.10±0.74 | 4.00±0.33 | 0.002** | ||
3.2.1.2. PRGF
KTW showed a statistically significant decrease after 3 months, and it decreased again at 6 months and then remained unchanged at 9 months. GT decreased slightly after 3 months and then increased again at 6 and 9 months. PD showed a constant and statistically significant decrease throughout the follow-up periods. Both MGI and MPI were found to be improved throughout the follow-up period.
3.3. Between-groups Result
No clinical parameters were found to be statistically significant, except PD, which was highly statistically significant at 6 months and statistically significant at 9 months.
The analyzed raw data of clinical assessments are tabulated in Table 6.
4. DISCUSSION
This study has been designed to evaluate the management of labial dehiscence at the time of immediate implantation in the esthetic zone using bio-membranes derived from the patient's own blood to exclude allergy and elicit immunity. Moreover, this process decreases the overall cost of the maneuver and assures autogenicity [45].
This study reported that both membranes were able to increase bone density while CGF induced more preservation of crestal bone and more amelioration of PD than PRGF.
This study discusses an important concern for most implantologists and reviews an economical approach utilizing autogenous biomaterial to manage a common problem faced by most of us while restoring the esthetic zone.
According to the Li-Chang 2021 classification of extraction sockets, we studied type II socket intact soft tissue wall with the destruction of at least one bone wall. According to the gingival and periodontal diseases classification of 2017, patients with gingival health with intact periodontium and those with gingival health with reduced periodontium are classified as non-periodontitis patients and stable periodontitis patients, respectively [46, 47].
Platelets are tiny, atypical cells (1.5–3 μm) derived from bone marrow. They can carry three different granules, which liberate multitudinous growth factors, including Platelet-Derived Growth Factor (PDGF), Vascular Endothelial Growth Factor (VEGF), Transforming Growth Factor (TGF), and Epidermal Growth Factor (EGF) [48].
Briefly, platelet derivatives can be widely subdivided into 4 major families and two generations depending on two main criteria (presence of leukocytes and density of fibrin). The first-generation preparations have a low density of fibrin, whether with leukocytes or without, while the second-generation preparations have a high density of fibrin (Platelet Rich Fibrin – PRF), whether with leukocyte (L) or without. Recently, CGF has been considered the third generation of platelet derivatives and an upgraded version of L-PRF as it contains more growth factors with denser fibrin matrix and leukocytes. It is also well-known that platelet concentrates have an increased level of heterogenicity because their nomenclatures are not standardized with the preparation protocol. This means that the reader must consider the preparation protocol mentioned in any study related to platelet derivatives and not rely only on one nomenclature [10, 49, 50].
PRGF membrane (P-PRP) is considered one of the gold standards in the field of platelet derivatives, and recent generations should be tested against it. Furthermore, CGF is considered relatively new in comparison to PRGF as CGF was introduced in 2006 by Sacco, while P-PRP preparations, such as PRGF, were introduced in the 1970s, and their preparation protocol has been modified many times to date. However, it has been proposed that CGF can facilitate bone growth and healing more than any older generation of platelet concentrates [51].
The main difference between these two membranes is that CGF contains leukocytes and complex three-dimensional fibrin architecture, while PRGF does not contain leukocytes and has lower fibrin density, so these two preparations are the extreme opposites concerning fibrin architecture and leukocyte content, providing a totally different pattern of growth factors release.
The CGF membranes continue releasing growth factors till the 7th day in large amounts, and most of those factors originate from the cell population of the membrane itself, while P-PRP resorbs after nearly three days and releases most of its growth factors in the first hours [21, 52-54].
It is obvious and worthy of notice that the handling and application of ready-to-use PRGF loaded with xenogeneic bone are easy but take a long time, which is nearly one hour from sample withdrawal to membrane insertion as the preparation process involves multiple and slightly complex steps, while on the other hand, CGF preparation consists of faster and less complex steps.
CBCT and its multifunction software have been used to ensure an accurate non-subjective measure of the effect of two membranes in terms of crestal bone loss and bone density as an indicator of the effectiveness of the membrane combined with xenogeneic to override the dehiscence [55].
Crestal bone loss around dental implants is an indispensable criterion for anticipating the durability of the implant. To be considered a successful implant, the first-year loss should be less than 1.5 mm. Our results indicated that both groups passed this criterion, while CGF showed much less crestal bone loss than PRGF [56, 57].
Crestal bone loss mainly occurs as a physiological part of the early healing process, and because CGF offers a higher release of growth factors, it can induce osteogenic differentiation of Human Bone Marrow Stem Cells (hBMSC) and promote endothelial angiogenesis due to the release of soluble and cellular components that promote the healing process significantly [58-60].
Our results concerning the advantageous use of CGF in decreasing crestal bone loss have been found to be in accordance with many studies, such as those of Karthik et al. and Sitamahlakshmi et al. [56, 61].
CGF expedites soft tissue healing as a barrier membrane; moreover, CGF is able to fill the jumping gap efficiently because of its higher fibrin tensile strength and stability due to agglutination of fibrinogen, factor XIII, and thrombin. So, when combined with bone graft, it is able to promote new bone formation [62].
The activity of the osseointegration process can be predicted by the density of newly formed bone around implants, bone strength, and resistance to micro and macro fractures. It is directly related to bone density, so lower bone density may hinder the loading of dental implants, making it an essential parameter for implant durability and successful loading with ideal stress distribution [63, 64].
The rate of increase in bone density was observed to be uniform for both membranes, rendering the intergroup comparison useless. However, our comparison of the two groups showed statistically significant results postopera- tively at 9 months.
S Manoj et al. recommended using CGF on immediate implants due to its intensifying effect on bone density, which has been found to be parallel to our results and those of other studies, such as studies by Shetty M et al., Inchingolo et al., and Yang L. et al. The studies have stated that CGF plays no significant role in promoting new bone regeneration when used alone without bone grafting [65-67].
We demonstrated decreased patient compliance and postoperative improvement in PD, MPI, and MGI in both groups, while the CGF group showed improvement in GT and KTW. It has been reported that CGF induces more proliferation and migration of Gingival Mesenchymal Stem Cells (GMSCs) and is able to promote the expression of pro-angiogenic and collagen-related proteins. Angio- genesis arises before the deposition of collagen and fibronectin, so it offers a generous blood supply and nutrients in the process of gingival development and repair. CGF enhances the expression of essential angiogenic factors of MSCs, such as VEGF and Ang-1, which are a sine qua non for neovascularization, thus coinciding with our soft tissue parameters [55, 39, 40, 41, 62].
It has also been reported by Harries et al. that the early-stage osteoblast differentiation, which is induced by CGF, is associated with type I collagen synthesis that may significantly promote soft tissue parameters [26, 68, 69].
Finally, bioactive and bioabsorbable membranes are recent evolutions that can be used as drug-releasing systems for growth factors that may notably improve bone and soft tissue around the immediate implants [69, 70].
CONCLUSION
The field of platelet derivatives is continuously growing and extensively investigated in many aspects of medicine and dentistry. Moreover, implantology is a very complex specialty with many factors that must be taken into consideration to achieve esthetic and functional success. Therefore, it is imperative to investigate the preparations of different platelet derivatives thoroughly to override the defect around dental implants and ensure maximum esthetic and functional success.
In our clinical trial, we investigated and compared the efficacy of two different preparations of platelet concentrates (CGF and PRGF) to manage labial dehiscence around immediate implants utilizing bony factors, including bone density and crestal bone loss and soft tissue factors, such as KTW, GT, PD, MGI, and MPI.
Concerning bony factors, our result suggests the superiority of CGF in the prevention of crestal bone loss and amelioration of bone density compared to PRG, while with respect to soft tissue factors, our results were not statistically significant except for between-group probing depth results, which showed more amelioration in CGF group.
Keeping the limitation of this study in mind, we advocate using CGF around immediate implants to manage labial dehiscence in the esthetic zone due to its easy and simple preparation method and the ability to decrease crestal bone loss and increase bone density.
LIMITATIONS AND RECOMMENDATIONS
It is recommended to conduct further studies with longer follow-up duration because the 9-month period was not enough in all cases to ensure complete stabilization and co-integration of the bone graft as some particles of the bone graft still remained in position at the 9-month follow-up. Moreover, more studies with a higher number of participants and type III socket in a block design are recommended to disclose the effect of platelet concentrate at different levels of bone dehiscence.
LIST OF ABBREVIATIONS
| IIP | = Immediate Implant Placement |
| P-PRP | = Pure Platelet-Rich Plasma |
| APCs | = Autologous Platelet Concentrates |
| CGF | = Concentrated Growth Factor |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was approved by the Minia University Faculty of Dentistry Ethics Committee (Approval Number: 86/2/560/2022).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
The informed consent form, as approved by the ethical committee, was signed by each patient.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.


