All published articles of this journal are available on ScienceDirect.
Evaluation of Soft Tissue Changes Following Platelet-rich Fibrin in Immediate Implantation of Posterior Teeth: A Randomized Clinical Trial
Abstract
Background:
Currently, immediate implant placement is favored due to its reduced number of surgical appointments and shorter overall treatment duration. However, a notable disadvantage of this protocol is the inadequate dimension of soft tissue surrounding the implant, which presents a challenge for immediate implantation in posterior teeth. As a result, patients frequently necessitate soft tissue augmentation to alleviate potential complications. In this randomized clinical trial study, a minimally invasive technique utilizing platelet-rich fibrin (PRF) was employed to enhance soft tissue thickness and keratinized tissue width during immediate implantation in the posterior region of the jaw.
Methods and Materials:
A total of 20 bone-level implants were utilized in 20 patients through the immediate implantation protocol. In order to augment soft tissue, 10 patients were subjected to treatment with platelet-rich fibrin (PRF) on immediate implants in the test group, while 10 patients received connective tissue graft (CTG) on immediate implants in the control group. After a period of 3 months, all implants were uncovered through surgery, and healing abutments were placed on fixtures. Keratinized tissue width was measured using a periodontal probe at baseline, 1 month, and 3 months after implant placement, while soft tissue thickness was measured at baseline and 3 months after implant placement.
Results:
Treatment was successful for all the patients. The average keratinized tissue width in the test and control group was 3.45 and 2.65 at baseline, 4.00 and 3.40 one month after surgery, and 4.20 and 3.85 three months after implant placement, respectively. Average keratinized tissue thickness in the test and control group was 2.45 and 2.05 at baseline and 3.20 and 3.25 at reentry (three months after implant placement), respectively. The average increase in keratinized tissue thickness from baseline to 3rd month was 0.75 in the test and 1.20 in the control group. The average increase in keratinized tissue width from baseline to 1st month was 0.55 in the test and 0.75 in the control group, and from baseline to 3rd month, it was 0.75 in the test and 1.20 in the control group, respectively. Data for both parameters in each group from baseline to 3rd month were statistically significant. Data for keratinized tissue thickness (p=0.077) and keratinized tissue width (p=0.089) between both the groups were not statistically significant.
Conclusion:
Platelet-rich fibrin (PRF) has the potential to serve as a minimally invasive approach for augmenting soft tissue in the posterior region of the jaw during immediate implant placement.
Clinical Trial Registration Number:
The clinical trial registration number for this study is IRCT20190723044313N1.
1. INTRODUCTION
Since 1982, after the introduction of osteointegration to the dentistry field by Dr. Per Ingvar Brånemark, treatment using implant therapy has become one of the main methods of treating edentulous patients [1].
The optimal timing for implant placement in relation to tooth extraction has been a topic of extensive discussion. The placement of implants can be categorized into three approaches, namely immediate, delayed, or staged. The determination of which approach to employ is contingent upon a multitude of factors, including the amount, quality, and support of existing bone, as well as the preferences of both the clinician and the patient. Immediate implant placement involves the placement of an implant at the time of extraction, while delayed implant placement is typically performed after a period of approximately 2 months to allow for soft tissue healing. Staged implant placement allows for significant bone healing within the extraction site and typically requires 4 to 6 months or longer. In recent years, immediate implant placement has emerged as a viable alternative approach to dental implant placement, which eliminates the need for a separate healing period prior to implant placement [2].
Following implant placement in the immediate implantation method, a gap is established between the implant and the socket wall [3]. The jumping gap term refers to the ability of bone to bridge the horizontal distance and fill the void. The thin buccal bone and the large jumping gap increase the risk of soft and hard tissue recession [4-6]. Therefore, it is better to fill the socket with bone replacements and augment the soft tissue at the time of implant surgery to prevent socket collapse and improve the biotype of the labial soft tissue [4, 7-11].
Immediate implants offer several advantages over the traditional approach, including reduced overall treatment time by eliminating the need for a separate healing period before implant placement [12-14], less surgical interventions, reduced cost, optimal availability of residual bone, soft tissue preservation, and reduced healing time. Some disadvantages of immediate implants are disruption of results due to thin biotype, the complication of the optimal placement and anchorage of the implant due to the post-extraction alveolar socket, potential lack of keratinized gingiva to accommodate the flap, possibility of additional surgery, technical sensitivity, lack of soft tissue required for implant coverage, the recession of soft tissue around the implant, and reduction of the facial bone after loading or during the healing period [2, 5].
Soft tissue interfaces with implants can take the form of either keratinized or non-keratinized mucosa. The former is firmly anchored to the underlying periosteum by collagen fibres, while the latter is characterized by the presence of elastic fibres that allow for its mobility over the underlying bone. The soft tissue relationship around implants is comparable to that of teeth, with a dense connective tissue layer separating the epithelial attachment and the marginal bone. This layer has limited vascularity in the immediate vicinity of the implant surface and is approximately 3 to 4 mm in total height, with the epithelial attachment comprising about 2 mm and the supracrestal connective tissue zone comprising about 1 mm. The thickness of peri-implant soft tissues can vary from 2 mm to several millimetres, as observed clinically. The soft tissue around implants and teeth share similar appearance and structural features, with the gingival/mucosal sulcus, junctional epithelium, and an area of connective tissue located on the supporting bone. Notably, the osseointegrated implant area lacks a periodontal ligament and collagen fibres. In this context, a human study has reported a biological width of 4 to 4.5 mm around the implant. The epithelium surrounding the implant is similar to that of a natural tooth and is located along the sulcular epithelium that covers the inner surface of the gingival sulcus [2]. The apical portion of the gingival sulcus is covered with junctional epithelium, also known as long junctional epithelium [15]. The morphology of the connective tissue around the implant is very similar to that of teeth, with the exception of the absence of cement, periodontal ligament, and inserting fibres. The connective tissue around the implant measures approximately 1-2 mm, which is longer than the periodontal connective tissue [16, 17]. Although the presence or absence of keratinized gingiva is not a prerequisite for long-term implant stability, an implant that is surrounded only by mucosa is more susceptible to problems around the implant [18].
To solve one of the problems of the immediate implant placement in the posterior area due to the inability to close the extracted tooth socket and decreased width of the keratinized gingiva, an intervention should be performed to provide complete coverage of the socket, increase the width of the keratinized gingiva, and finally improve the success rate of the immediate implantation.
The gold standard technique is to place connective tissue graft (CTG) on the socket as a biological membrane. The wound left to secondary healing in the patient's mouth is one of the disadvantages of this method. An alternative method is soft tissue augmentation with platelet-rich fibrin (PRF) [19, 20].
PRF, first introduced by Choukron (2000), is the second generation of platelet concentrate, which contains the highest concentration of platelets, growth factors [vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-β (TGF)], fibrin, fibronectin, and thrombospondin. This technique is simple and does not require thrombin. According to leukocyte content and fibrin structure, there are four categories of platelet concentrate [2], four of which are as follows:
• Pure platelet-rich plasma (P-PRP) or leucocyte-poor PRP products are preparations without leucocytes and with a low-density fibrin network after activation.
• Leucocyte- and platelet-rich plasma (L-PRP) products are preparations with leucocytes and a low-density fibrin network after activation.
• Pure platelet-rich fibrin (P-PRF) or leucocyte-poor platelet-rich fibrin preparations are without leucocytes and with a high-density fibrin network.
• Leucocyte- and platelet-rich fibrin (L-PRF) or second-generation PRP products are preparations with leucocytes and a high-density fibrin network.
Applications of L-PRF include extra-oral applications [treatment of diabetic foot ulcers (DFUs), pressure ulcers (PUs), acute surgical wounds, and venous leg ulcers (VLUs)], treatment of periodontal bone lesions, ridge preservation, mucogingival surgery, sinus floor augmentation, implant surgery, medication-related osteonecrosis of the jaw (MRONJ), and PRF-block.
Since L-PRF has a strong fibrin network, it is used as a membrane and soft tissue graft. L-PRF slowly releases growth factors and matrix proteins over 7 days. These processes accelerate wound closure and healing, and in the long term increase the stability of soft tissue coverage and the thickness of the gingiva [2, 21].
The present study aimed to investigate the efficacy of utilizing a less invasive platelet-rich fibrin (PRF) membrane as a substitute for soft tissue grafts in achieving full coverage of the extracted tooth socket, alleviating pain and discomfort, augmenting the thickness and width of the keratinized gingiva, and enhancing the success rate of immediate implants.
2. MATERIALS AND METHODS
A randomized clinical trial with a concurrent parallel study design was conducted to examine the effectiveness of platelet-rich fibrin (PRF) as a substitute for enhancing soft tissue around dental implants on the posterior region of the jaw. The study population consisted of 20 patients who were randomly assigned to two groups (test and control) based on predetermined inclusion and exclusion criteria. Participants were selected from patients who sought dental treatment at Mashhad Dentistry Faculty in Iran between 2018 and 2019. The randomization process was accomplished through the use of sealed envelopes at the time of surgery.
Initially, patients were selected by inclusion criteria, which were good oral hygiene (plaque index <20%), partially edentulous jaw on posterior region (Kennedy Class I, II, and III), sufficient bone at the implant site, no need for periodontal surgery around the site, and stable occlusion of patient's dentition. Exclusion criteria were infection around the implant site, history of a bleeding disorder or anticoagulant therapy, immunocompromised patients or those with a disabling disorder, severe systemic disease, being a tobacco user (more than 15 per day), and drug or alcohol addiction.
After explaining the practical process of the research to the participants, informed patient consent was obtained. All participants in the study received standard treatment with the same implant system (bone-level implant). Randomization of individuals was done by the random block method.
The participants were divided into two groups as follows:
1. The test group: prf as membranes + mineralized bone allograft powder 500-1000µ (Regen allograft, Iranian tissue product company, Tehran, Iran).
2. The control group: connective tissue without prf + mineralized bone allograft powder 500-1000µ (Regen allograft, Iranian tissue product company, Tehran, Iran).
2.1. Preoperative Phase
During this phase, several procedures were carried out to prepare the patients for the implant placement procedure. One of the primary procedures performed during this phase was the scaling of all of the patients’ teeth. This involved the removal of plaque and calculus from the teeth to ensure optimal oral health. Additionally, oral health instructions were provided to the patients to maintain good oral hygiene in preparation for the implant placement. Following four weeks of maintenance, the patients underwent immediate implant placement.
2.2. Surgical Technique
The first step was atraumatic extraction of the posterior tooth using a 1.8 ml dental cartridge containing lidocaine hydrochloride 20mg/epinephrine 12.5 mcg as a local anaesthesic (Xylopen 2%, Exir Pharmaceutical Company, Tehran, Iran) (Fig. 1).
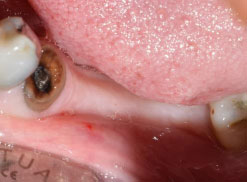
The atraumatic extraction of the tooth was achieved using a periotome, which involved inserting the metal blade into the periodontal ligament and circumferentially separating the majority of Sharpey's fibers from the root surface. Dental forceps were then used to rotate the tooth and facilitate its easy removal with minimal pressure. For teeth that were multirooted or had complex anatomical features, such as curves, sectioning with a high-speed bur was done to create smaller, manageable pieces for extraction. Once the tooth has been sectioned, each piece was carefully extracted with minimal pressure to avoid damage to surrounding tissues.
During the extraction procedure, it is imperative to provide meticulous attention to both the preservation and safeguarding of the buccal bone. Furthermore, the debridement of periapical lesions of the extracted socket is also of utmost importance [22-24].
The surgical phase consisted of the full-thickness mucoperiosteal flap with the preparation of the osteotomy site in both groups. The implant bed was prepared according to the standard protocol using the starter drill (diameter enhancer), spiral drills (internally cooled drill), and normal saline.
The implant with a diameter of 4.1 mm or 4.8 mm and the maximum allowable length was inserted inside the socket of the extracted tooth. Indeed, to maintain primary stability, it was inserted in the ½ or 2/3 of the lingual and apical sides of the extraction socket (Fig. 2). The implant platform was placed approximately 3-4 mm more apical to the mid-buccal position of the anticipated marginal mucosa of the future implant crown. in addition, the implant was positioned 1 mm more towards the palatal direction compared to the protrusion of the prosthesis, with respect to the buccolingual position, and the coverscrew was connected to the fixture.
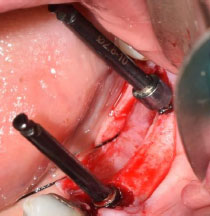
After implant placement, the distance between the implant and the bony wall of the socket (jumping distance) in both groups was filled with allografts (Fig. 3). For wound closure, dehiscence repair, and an increase in keratinized tissue on the socket, connective tissue graft and PRF were, respectively, used in the control and test groups after immediate implant placement.
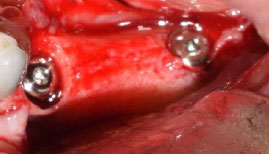
In the control group, connective tissue was harvested from the hard palate (the area between the first premolar, first molar, and 2 mm apical to the gingival margin), maxillary tuberosity, and edentulous ridge. The thickness of the acquired connective tissue was approximately 1 mm. The harvested tissue was placed on the implant and under the residual keratinized mucosa of buccal and lingual regions of the surgical site and then fixed with polyglactin absorbable suture (SUPABON, SUPA Medical Devices Company, Tehran, Iran).
2.3. Platelet-rich Fibrin Preparation
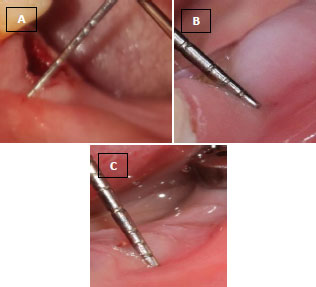
In the operating room, right before surgery, 10 ml of blood was taken from the antecubital vein of the patients and transferred into a test tube without anticoagulant. Blood samples were immediately (in less than 60 s) centrifuged at 2700 rpm for 12 min, and then the PRF clot was placed in an Xpression kit to apply gentle pressure by gravity and obtain a strong fibrin membrane. In less than 2 h from the formation of the membranes and before the wound closure, they were placed on the surface of the implant (Fig. 4A-C).
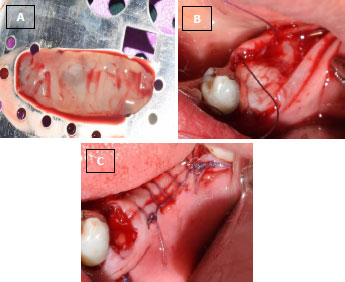
(B) L-PRF membrane placed on the site of the immediate implant.
(C) Sutured flap and exposed L-PRF membrane on the implant.
After surgery, the patient was asked to continue taking amoxicillin 500 mg (Farabi Pharmaceutical Company, Isfahan, Iran) three times a day for 5 days and ibuprofen 400 mg as an analgesic agent (Gelofen, Dana Pharmaceutical Company, Tabriz, Iran) three times a day for two days. Postoperative instructions were given to the patient. Chlorhexidine 0.02% mouthwash was prescribed for four weeks.
The suture was removed ten days after the surgery and the site was rinsed with saline. The surgical site was then evaluated. Three months after the operation, the implant was exposed using the flap technique, and the required healing abutment was placed on the fixture to form a suitable profile for the implant. Soft tissue changes were assessed at baseline, one month, and three months after implant placement with the Williams periodontal probe (North Carolina Hu-Friedy, Chicago, IL, USA).
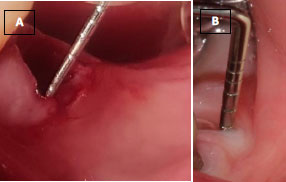
(B) Keratinized gingival width one month after the surgery.
(C) Keratinized gingival width three months after the surgery.
Measurable parameters included the following:
• Keratinized gingival width (baseline, one month, and three months after implant surgery) (Fig. 5A-C).
• Keratinized gingival thickness (baseline and three months after implant surgery) (Fig. 6A, B).
(B) Keratinized gingival thickness three months after the surgery.
2.4. Method of Data Collection
2.4.1. Statistical Methods
All the data obtained were analyzed using the SPSS, version 22. The normal distribution of data was verified using the Shapiro-Wilk test. Repeated measures ANOVA and Bonferroni post-hoc test were used to check for significant differences in keratinized gingival width between times. A paired t-test was used to check significant differences in keratinized gingival thickness between times and an unpaired t-test was used to check a significant difference in the keratinized gingival thickness and width, as well as their changes between the groups. The significant level was set at p < 0.05.
3. RESULTS
A total of 20 individuals, consisting of 10 females and 10 males, with a mean age of 39.25 ± 7.95 years and an age range of 27 to 58 years, were enrolled in this investigation. The PRF and CTG groups were observed to exhibit no discernible differences in age and gender (p=0.497 and p=0.371, respectively). The average width of keratinized gingiva at baseline, one month, and three months was not deemed statistically significant between the two groups (p=0.143, p=0.393, and p=0.579, respectively). In the PRF group, the width of the keratinized gingiva demonstrated statistical significance across time (p=0.004). Specifically, the keratinized gingival width increased significantly after three months compared to baseline. The observed changes in the width of keratinized gingiva were found to be statistically significant in the CTG group over time (p<0.001). The keratinized gingival width was significantly increased at every time point compared to the previous time point (Table 1).
Table 2 presents the findings of the study on the average thickness of the keratinized gingiva at baseline and after three months, where no significant differences were observed between the two groups (p=0.123 and p=0.870, respectively). However, it is noteworthy that both the PRF and CTG groups demonstrated a statistically significant increase in gingival width three months post-treatment compared to the baseline (p=0.017 and p=0.004, respectively).
| Group | Baseline | 1 Month | 3 Months | p-value£ |
|---|---|---|---|---|
| PRF | 3.45±1.21a | 4.00±1.15a,b | 4.20±1.03b | 0.004ϕ |
| CTG | 2.65±0.88a | 3.40±0.99b | 3.85±0.94c | <0.001 ϕ |
| P-value¥ | 0.143 | 0.393 | 0.579 | - |
| Group | Baseline | 3 Months | p-value£ |
|---|---|---|---|
| PRF | 2.45±0.50a | 3.20±0.59b | 0.017ϕ |
| CTG | 2.05±0.60a | 3.25±0.75b | 0.004 ϕ |
| P-value¥ | 0.123 | 0.870 | - |
The present study involved the calculation of alterations in both the width and thickness of keratinized gingiva at each time point in relation to preceding time points. These changes were subsequently compared between the two groups receiving either platelet-rich fibrin (PRF) or connective tissue graft (CTG). The results indicated no statistically significant differences in the changes observed in the width of keratinized gingiva between the two groups, as presented in Table 3.
| Variables | Width | Thickness | ||
|---|---|---|---|---|
| Group | A | B | C | C |
| PRF | 0.55±0.69 | 0.20±0.42 | 0.75±0.63 | 0.75±0.68 |
| CTG | 0.75±0.59 | 0.45±0.60 | 1.20±0.48 | 1.20±0.35 |
| P-value¥ | 0.383 | 0.300 | 0.089 | 0.077 |
4. DISCUSSION
Platelet-rich fibrin (PRF) is a second-generation platelet concentrate obtained through the centrifugation of autogenous blood. It is characterized by high concentrations of platelets, growth factors (VEGF, PDGF, and TGF), fibrin, fibronectin, and thrombospondin, which can accelerate the regeneration of soft tissue and bone [25].
The present study aimed to evaluate the efficacy of PRF for soft tissue augmentation during immediate posterior tooth implantation. Patients who met the inclusion criteria were divided into two groups: the test group, in which PRF was placed on the implantation socket, and the control group, in which connective tissue graft (CTG) was used.
The results of the study indicated that the use of PRF for soft tissue augmentation during immediate posterior tooth implantation was appropriate and comparable to the use of CTG. Specifically, the keratinized gingival width using L-PRF increased by 0.55 mm from the baseline to the first month and 0.20 mm from the first to the third month at the healing placement time. Overall, the keratinized gingival width increased by an average of 0.75 mm from the baseline to the third month. In the control group, the mean increase in keratinized gingival width was 1.20 mm. Moreover, the thickness of soft tissue increased from 2.45 to 3.20 mm (0.75 mm on average) from the baseline to the time of the second stage of surgery in the test group. In contrast, the increase in the control group was 1.20 mm on average.
The available literature on the use of platelet-rich fibrin (PRF) for the augmentation of soft tissue around implants is limited, with only a few studies having been conducted [20, 26, 27]. Furthermore, the results of these studies are not consistent with each other or with the findings of the present study. For instance, Temmerman et al. [20] used a different technique, namely free gingival graft (FGG), and reported that PRF is effective in augmenting soft tissue around implants but has a lesser effect than FGG. In a subsequent study, they found that both L-PRF and FGG increased gingival width, but the increase was greater in the FGG group (1.3 ± 0.9 mm) (P <0.05).
In contrast, Hehn et al. [26] used PRF with the split-flap technique to increase mucosal thickness and found that it did not affect soft tissue augmentation around implants, which is contrary to the present study's findings. In some studies similar to the present one, Eren, Tunali, and Aleksic [28-30] reported that PRF had a similar effect to connective tissue graft (CTG) on soft tissue augmentation, but they examined the soft tissue around teeth and implants using the coronally advanced flap method to resolve gingival recession.
Jankovic et al. [31, 32], on the other hand, found that PRF had a positive effect on soft tissue augmentation around teeth, but its effect was less significant than that of soft tissue graft. They also reported that there was more repair in the PRF group [31]. In a 2010 study, they compared the use of PRF with enamel matrix derivative (EMD) for soft tissue augmentation around teeth and found no significant differences between them [32].
Increasing the thickness of soft tissues around implants is beneficial for several reasons. Lindhe and Berglundh [16] and Linkevicius et al. [33] reported that when soft tissue is thin, crestal bone will be depleted to compensate for the required soft tissue thickness. Therefore, thick tissues around implants allow the formation of biological width without crestal bone recession. In the present study, the thickness of soft tissue was examined before placing the healing abutment, and there was no possibility of increasing it as a result of bone resorption to establish the biological width.
In the control group of the present study, CTG was used as the gold standard technique for soft tissue augmentation, which is consistent with many other studies [34-37]. Overall, this study suggests that PRF can be a viable alternative to CTG for soft tissue augmentation around immediate implants in the posterior region of the jaw, but more studies are needed to confirm its long-term efficacy.
CONCLUSION
The findings of this investigation suggest that the minimally invasive L-PRF technique may be employed to enhance the thickness and width of the keratinized gingiva during immediate implant placement in the posterior region of the jaw. This approach yielded an average augmentation of 0.75 mm (P = 0.077) in keratinized gingival thickness and 0.75 mm (P = 0.089) in keratinized gingival width.
LIST OF ABBREVIATIONS
| CTG | = Connective tissue graft |
| PRF | = Platelet-rich fibrin |
| VEGF | = Vascular endothelial growth factor |
| PDGF | = Platelet-derived growth factor |
| TGF | = Transforming growth factor-β |
| DFU | = Diabetic foot ulcer |
| Pus | = Pressure ulcers |
| VLU | = Venous leg ulcers |
| MRONJ | = Medication-related osteonecrosis of the jaw |
| PPP | = Platelet-poor plasma |
| RBC | = Red blood cells |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research was presented to the Organizational Ethics Committee of the Faculty of Dentistry, Mashhad University of Medical Sciences on 2019-12-25 with the number 640, and was approved under the code IR.MUMS.DENTISTRY. REC.1398.080.
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. The reported experiments were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All patients provided an informed consent form and were assured of the confidentiality of the data.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study will be available from the corresponding author, [M.J], on special request.
CONFLICT OF INTEREST
Dr. Amir Moeintaghavi is an editorial board member of The Open Dentistry Journal.
ACKNOWLEDGEMENTS
Declared none.


