All published articles of this journal are available on ScienceDirect.
Verrucous Carcinoma, A Very Well Differentiated Form of OSCC? - An Immunochemical Exploratory Study
Abstract
Introduction:
Verrucous carcinoma shows an analogous biological behaviour to well-differentiated Oral Squamous Cell Carcinoma (OSCC). So, it is a difficult task to differentiate the above two entities based on clinical and pathological behaviour. Immunohistochemical studies can help in investigating the differences between the biological behaviours of these carcinomas. Laminin may be used as a status indicator of intact or degraded basement membrane during tumorigenesis. Thus, the present study aimed to assess the immunohistochemical expression of laminin in different grades histological grades of OSCC and VC.
Methods:
Laminin expression was evaluated in 31 biopsy-proven cases of Oral Squamous Cell Carcinoma (OSCC) and 10 cases of Verrucous Carcinoma (VC).
Results:
We observed that most cases of VC (90%) were negative or mildly stained for cytoplasmic laminin while the majority of cases of OSCC (74.2%) stained moderately for the same. Further, VC cases showed a total absence of linear laminin staining around the tumour-host interface in contrast to 13% of OSCC cases that were correspondingly stained (p<0.001).
Conclusion:
The present study, therefore concludes that the interface expression in Verrucous Carcinoma distinctly differed from that in well-differentiated Oral Squamous Cell Carcinoma. Cytoplasmic laminin expression increased and interface staining decreased with unfavourable clinicopathological outcomes thereby rendering laminin an expedient marker in evaluating the histological differentiation and aggressiveness of oral carcinoma
1. INTRODUCTION
Oral squamous cell carcinoma is one of the most invasive human tumours with high mortality and morbidity. However, Ackerman first described Verrucous Carcinoma (VC), a highly differentiated variant of Squamous Cell Carcinoma (SCC), in 1948 [1] and reported its different clinical behaviour from classical Oral Squamous cell Carcinoma (OSCC). The tumour is slow-growing, locally destructive, and invasive. It clinically presents as a relatively asymptomatic, well-demarcated, whitish exophytic growth. Histologically, it has a broad base and pushing margins and the presence of an intact Basement Membrane (BM) distinguishes it from OSCC [2, 3]. As verrucous carcinoma has similar biological behaviour to OSCC, it is difficult to differentiate the above two just based on clinical and pathological behaviour. Further to avoid mishaps in the clinical diagnosis and treatment of OVC and OSCC, it is of utmost importance to differentiate the two concerning their different molecular mechanisms and prognosis [4]. With this regard, molecular approaches such as immuno-histochemical studies can help in investigating the differences between biological behaviours of these carcinomas [5].
The epithelium is separated from the underlying stroma by a thin basement membrane (BM). BM not only modulates the cellular function but also serves as a stout structural barrier against tumour invasion. In the course of tumour progression, the BM undergoes important quantitative and qualitative changes and thus, its destruction represents the first step in invasion and metastasis [6]. It has been hypothesized that the tumour cells attach to the BM through the cell-surface receptors and in turn bind precisely to matrix components such as laminin (a 900kDa mosaic BM glycoprotein) and this binding is a prerequisite for BM degradation and tumour invasion. Thus, as a readily detectable BM component, laminin may be used as a status indicator of intact or degraded basement membrane during tumorigenesis [7].
As far as our search, very few studies have been conducted correlating the role of laminin to verrucous carcinoma and also demonstrating the correlation of laminin, with OSCC, and VC.
Thus, the present study aimed to assess the immunohis-tochemical expression of laminin in different grades histo-logical grades of OSCC and VC.
2. MATERIALS AND METHODS
This retrospective immunohistochemical study was con-ducted in the Department of Oral Pathology and Microbiology, Manipal College of Dental Sciences, Mangalore. Clearance from the institutional ethics committee (ref number 13137) was obtained before the commencement of the study.
Formalin-fixed, paraffin-embedded tissue blocks of 31 biopsy-proven cases of Oral Squamous Cell Carcinoma (OSCC) and 10 cases of verrucous Carcinoma (VC) were retrieved from departmental archives. The inclusion criterion defined patients with primary OSCC and VC, who underwent curative excision as the primary mode of treatment to be included in the study. Exclusion criteria were patients who underwent radiotherapy and chemotherapy as the primary mode of treatment as these forms of treatment are said to release enzymes like (Matrix Mettaloproteinases) MMP that indirectly affect the expression of laminin.
2.1. Scoring Methodology for Laminin Expression
2.1.1. Technique for Evaluation
Ten consecutive representative fields at invasive tumour front (ITF) were examined in both 10x and 40x in each case of OSCC using Bryne’s grading system (1992) (Bryne et al., 1989) [8] at the Invasive Tumour Front. A score between 1 and 4 was given for each of the five parameters of the Brynes grading system and summated to obtain a total score for each case. Cases of OSCC with scores between 5 and 8 were classified as well-differentiated cases, 9 to 12 as moderately-differentiated and above 13 as poorly-differentiated cases. The H&E-stained sections of Verrucous Carcinoma were re-examined to confirm the lack of invasion. Immunostaining was performed using laminin and secondary reagents using super sensitive IHC detection system (BioGenex Laboratories Inc. 49026, Catelogue number QD420-YIKE Milmont Drive, Fremont, CA 94538, USA). For comparison, normal buccal mucosa was the external staining control and normal blood vessels in the tissue sections were the internal positive control.
Using the modified method proposed by Ono et al., (1999) [9], the cytoplasmic staining of laminin in the tumour cells was graded as follows:
| 3 | Intensity similar to that of control |
| 2 | Lesser intensity as the control but discernible cytoplasmic staining |
| 1 | Mild staining |
| 0 | No stain |
Further, the pattern of laminin staining around the islands (at the tumour host interface) was graded using the modified method proposed by Arduino et al., (2010) [10] which were graded as follows.
| 3 | Continuous linear staining with definite colour |
| 2 | Linear staining with moderate colour |
| 1 | Weak staining |
| 0 | Absent or very weak staining |
2.2. Statistical Analysis
Laminin expression at the cytoplasm and the epithelial connective tissue interface were compared using the chi-square test (Fisher’ exact test) between the various grades of OSCC and VC (Table 1). SPSS statistical software package version (20.0) was used and a p-value of 0.05 was considered as the level of significance.
| - | Well Differentiated | Moderately Differentiated | Poorly Differentiated | Verrucous Carcinoma |
Chi-square (P value) for grades of OSCC and VC |
Total OSCC | Chi-square (Pvalue) for Total OSCC vs VC | |
|---|---|---|---|---|---|---|---|---|
| Cytoplasmic laminin in peripheral cells | Mild or no presence of laminin | 1(8.33) | 1(9.09) | 0(0) | 9(90) | 38.49 (< 0.001) | 2(6.45) | 23.36 (<0.001) |
| Moderate presence of laminin | 11(91.67) | 10(90.91) | 2(25) | 1(10) | 23(74.19) | |||
| Intense presence of laminin | 0(0) | 0(0) | 6(75) | 0(0) | 6(19.35) | |||
| Cytoplasmic laminin in central cells | Mild or no presence of laminin | 11(91.67) | 1(9.09) | 1(12.5) | 3(30) | 36.261 (<0.001) | 13(41.94) | 3.809 (0.149) |
| Moderate presence of laminin | 1(8.33) | 10(90.91) | 1(12.5) | 7(70) | 12(38.71) | |||
| Intense presence of laminin | 0(0) | 0(0) | 6(75) | 0(0) | 6(19.35) | |||
| Pattern of laminin | No or very weak staining | 7(58.33) | 11(100) | 8(100) | 10(100) | 38.49 (< 0.001) | 26(83.87) | 38.49 (< 0.001) |
| Weak staining | 1(8.33) | 0(0) | 0(0) | 0(0) | 1(3.23) | |||
| Linear staining, moderately coloured | 2(16.67) | 0(0) | 0(0) | 0(0) | 2(6.45) | |||
| Continuous linear staining, definitely coloured | 2(16.67) | 0(0) | 0(0) | 0(0) | 2(6.45) | |||
| - | -
Cytoplasmic Staining Periphral/Central |
Linear Staining | ||
|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | |
| Sensitivity | 37.50% | 15.20% to 64.57% | 33.33% | 11.82% to 61.62% |
| Specificity | 100.00% | 86.28% to 100.00% | 100.00% | 86.77% to 100.00% |
| Positive Likelihood Ratio | - | - | - | - |
| Negative Likelihood Ratio | 0.62 | 0.43 to 0.91 | 0.67 | 0.47 to 0.95 |
| Positive Predictive Value (*) | 100.00% | 100.00% | ||
| Negative Predictive Value (*) | 71.43% | 63.11% to 78.51% | 72.22% | 64.51% to 78.81% |
| Accuracy (*) | 75.61% | 59.70% to 87.64% | 75.61% | 59.70% to 87.64% |
3. RESULTS
Comparison of laminin expression between different grades of OSCC and Verrucous Carcinoma revealed significant differences between the groups (p=<0.001). Mild to absent cytoplasmic laminin staining was seen in the majority of the cases (90%, n=9) of VC while most of the better-differentiated forms (90.91, n=11) of moderately differentiated squamous cell carcinoma (MDSCC) and 91.67% (n=10) of WDSCC) showed moderate staining. Intense cytoplasmic staining was seen only in 75% of cases of PDSCC. All cases of VC, MDSCC and poorly differentiated squamous cell carcinoma (PDSCC) were negative for laminin stain at the tumour-host interface while only 33.34% (n=12) of WDSCC showed linear staining around the tumour host interface (p=<0.001).
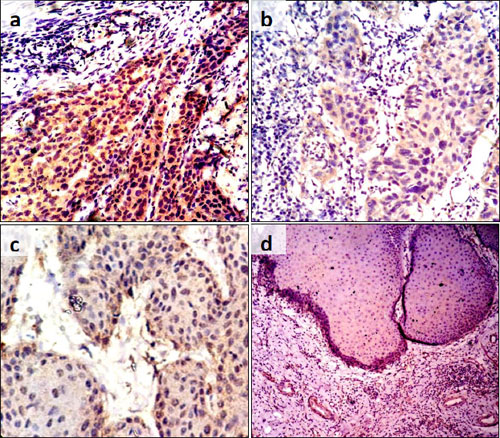
Most cases of VC (90%; n=9) were negative or mildly stained for cytoplasmic laminin while the majority of cases of OSCC (74.19%, n=23) stained moderately for the same. Further, VC cases showed a total absence of linear laminin staining around the tumour-host interface in contrast to 12.9% (6.45%,n=2 moderately coloured and 6.45%,n=2 well defined) of OSCC cases that were correspondingly stained (p<0.001). (Fig. 1a-d).
The presence of cytoplasmic staining or linear (weak to intense) staining of laminin at the tumour host interface showed an overall accuracy of 75.61% in differentiating OSCC from VC. The specificity ranged from 86.28% to 100% and 86.77% to 100%, respectively for cytoplasmic staining and linear staining of interface to predict oral squamous cell carcinoma. The positive predictive values were 100% and negative predictive values were 71.43% and 72.44%, respectively for cytoplasmic staining and linear staining (Table 2).
To summarize, the expression of laminin is distinctly different in VC and OSCC. The cytoplasmic expression of laminin was largely absent or mild in lesional cells in VC while being predominantly moderate to intense as the grade of OSCC worsens. Moderate to definite linear staining of laminin was visible only in 33.34% of cases of WDSC.
4. DISCUSSION
The heterogeneity of cancer is manifested by the interplay of the ever-increasing genetic and epigenetic changes that disturb cell regulatory networks and alter the interaction of cells with their microenvironment. Normally, cell-to-extracellular matrix (ECM) interactions are modulated by ECM ligands and receptors and by the ordered assembly of ECM molecules. Invasion by a tumour and its further progression require disruption of the ECM and degradation of the basement membrane (BM) enabled by the tumour cells themselves and their products.
The present study evaluated the role of laminin in the pathology of Oral Squamous Cell Carcinoma (OSCC) and Verrucous Carcinoma by correlating its expression.
In our previous study, we assessed the different grades of OSCC cases for the laminin expression at the tumour interface and within the cytoplasm of tumour cells. It was observed that the linear staining pattern at the tumour interface was mostly seen in WDSCC cases, while intense cytoplasmic expression within tumour cells was seen in PDSCC cases. A vogue of higher cytoplasmic laminin expression in tandem with weak/absent linear staining of laminin around the tumour-host interface was observed in OSCC cases with metastatic lymph nodes and involved surgical margins. Similarly, in most of the recurrent OSCC cases and cases of death tumour-host interface exhibited weak/absent linear staining of laminin whereas within tumour cells there was moderately intense cytoplasmic laminin expression. Therefore, these features give an insight into the role played by laminin in inducing cell migration and metastasis by regulating the formation of lamellipodia as well as its interactions with integrins, proteases, EGFR and various signalling pathways. Thus, oral carcinomas exhibit characteristic high-affinity sites on the cell surface for binding with laminin and may act as ligand/ laminin receptors for invasion (Yellapurkar, et al. 2018).
VC has been considered as a well-differentiated variant of OSCC. Laminin expression was shifted from the epithelial interface to cytoplasmic from well to poorly differentiated SCC. Following this trend, we were expecting to see an interface staining pattern of laminin in VC. This finding indicates innate molecular and genetic differences seen between WDSCC and VC. Wang et al., (2014) suggested certain qualitative and quantitative differences in WDSCC and VC to account for the differences in the clinical behaviour and progression between the two groups. These variations may be in type and amount of protein (in particular, basement membrane elements including laminin, laminin-5, collagen IV, and fibronectin) as well as gene expression (αB-crystallin, MMP-2, MMP-9, vascular endothelial growth factor). The findings by Wang et al. and our observations of a difference in the pattern of laminin staining at the basement membrane lead us to question the established and popular concept of Verrucous Carcinoma being a well-differentiated form of Oral Squamous Cell Carcinoma [11, 12].
Expression of the cytoplasmic laminin in the central and peripheral cells, showed different patterns in VC and well-differentiated OSCC making it a less useful parameter to differentiate the two. OSCC cases showed linear staining at the tumour-host interface which was not seen in VC. This indicates that VC has a different molecular signature compared to OSCC in terms of laminin expression.
An unequivocal differentiation of OSCC from VC is clinically pertinent. Incisional biopsy specimens with a histopathological diagnosis of verrucous carcinoma may still have foci of invasion in another part of the specimen. These entities have been named “verruco-squamous carcinoma” and have the squamous cell carcinoma element, rather than VC that defines the treatment [1]. Moreover, cervical lymphadenopathy which sometimes can be associated with VC, represents reactive changes and not a metastatic disease, and thus neck dissection is not indicated. According to the various literature reviews, radiation therapy is contraindicated in verrucous carcinoma as there will be a manifestation of radiation-induced anaplastic transformation, following the radiotherapeutic cycle. Therefore, it should be entailed that VC should be correctly diagnosed and differentiated from the classical OSCC [13].
CONCLUSION
The present study, therefore concludes that the expression of laminin varies between the grades of OSCC, being predominantly localized to the cytoplasm in poorly-differentiated cases and showing distinct interface staining with improving histological grades. The interface expression in Verrucous Carcinoma distinctly differed from that in well-differentiated Oral Squamous Cell Carcinoma.
Increased cytoplasmic expression of laminin and the presence of linear staining is more indicative of oral squamous cell carcinoma. The differential expression of laminin gives an insight into the differing pathogenesis of VC and OSCC. Further, this pattern of expression can be used as an expedient marker in evaluating histological differentiation and classification of OSCC/VC.
LIST OF ABBREVIATIONS
| ITF | = Invasive Tumour Front |
| VC | = Verrucous Carcinoma |
| OSCC | = Oral Squamous Cell Carcinoma |
| BM | = Basement Membrane |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This retrospective immunohistochemical study was con-ducted in the Department of Oral Pathology and Microbiology, Manipal College of Dental Sciences, Mangalore. Clearance from the institutional ethics committee (ref number 13137) was obtained before the commencement of the study.meth.
HUMAN AND ANIMAL RIGHTS
No human or animals were used for the studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information is available within the article.
FUNDING
None
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


