All published articles of this journal are available on ScienceDirect.
Effect Of Nano-Bioactive Glass On Flexural Strength And Antimicrobial Activity Of Resin-Modified Glass Ionomer Cement Containing 58S Nano-Bioactive Glass
Abstract
Introduction:
Bioactive glass (BAG) is increasingly used in dentistry, aiming to provide superior mechanical properties, optimal chemical stability, and favorable antimicrobial activity in the oral environment. This study aimed to measure the flexural strength (FS) and antimicrobial activity of resin-modified glass ionomer (RMGI) cement containing 58S nano-BAG.
Materials and Methods:
In this in vitro study, 0wt (Weight) %, 10wt%, 20wt%, and 30wt% 58S nano-BAG particles were added to RMGI powder in groups 1 to 4, respectively (n=10). Forty specimens were fabricated in metal molds (2 x 25 x 2 mm), and their FS was measured by using a three-point bending test at a crosshead speed of 0.5 mm/min. The antibacterial activity of the materials against Streptococcus mutants was assessed by the disc diffusion test. In addition to the abovementioned experimental groups, one control group (n=10) containing 100% BAG was also considered. Data were analyzed by one-way ANOVA and Tukey’s test.
Results:
The mean (± standard deviation) FS was 38.71±8.84, 43.61±17.34, 45.62±15.89, and 54.71±14.25 MPa in groups 1 to 4, respectively. No significant difference was noted in FS among the groups (P=0.06). A significant difference was found in the diameter of the growth inhibition zone among the groups (P<0.05), and group 4 containing 30wt% BAG showed minimal bacterial growth.
Conclusion:
The addition of 10wt%, 20wt% and 30wt% nano-BAG to RMGI powder did not significantly change the FS but the addition of 30wt% nano-BAG to RMGI significantly inhibited the bacterial growth.
1. INTRODUCTION
Materials used in the human body should be chemically stable and biocompatible, and the oral cavity is no exception to this rule [1]. Amalgam, composite resin, and most dental cements possess the abovementioned properties [2]. The first idea regarding the production of bioactive materials for use in the human body originated from the process of fluoride release from dental materials. Fluoride release by dental materials such as Glass ionomers has beneficial effects [2].
Glass ionomers are extensively used in restorative dentistry. One major advantage of glass ionomers over other restorative materials is that they do not require bonding for clinical durability. Also, they have optimal biocompatibility and do not irritate the dental pulp [3, 4]. Although glass ionomers are routinely used as dental cements, they have some shortcomings as well. The most important shortcoming of glass ionomers is their inadequate strength and toughness. In order to improve the mechanical properties of glass ionomers, the resin was added to glass ionomer powder to create resin-modified glass ionomer (RMGI) cements. RMGI contains hydrophilic monomers and polymers (HEMA), and has higher flexural strength (FS) than the conventional glass ionomers [5]. Flexural tests are widely used in dental research for composites [6] and acrylic resins [7]. Also, GICs can be tested accordingly in order to evaluate a fundamental mechanical characteristic.
However, the addition of HEMA decreases the fluoride release and, subsequently the bioactivity and enamel remineralization potential [8]. Thus, any modification that can increase the bioactivity of RMGI without decreasing its mechanical properties would be favorable [9]. Bioactive glass (BAG) has a reactive surface and is made of minerals such as sodium, phosphorus, calcium, silicate, etc. In a physiological environment, BAG can bond to the bone and some soft tissues [10]. Addition of bioactive glass (BAG) to the formulation of glass ionomer enhances its bioactivity and remineralization potential [3, 5-11].
Bacterial leakage and proliferation of microorganisms at the tooth-restoration interface is one major reason for the failure of dental cements. Evidence shows that BAG particles have antimicrobial properties [12]. On the other hand, RMGI has fluoride release potential [13, 14]. Thus, the addition of any material to increase the antimicrobial properties of RMGI with no adverse effect on its mechanical properties would be highly favorable [15]. To the best of the authors’ knowledge, there is no study about adding 58S nano-BAG to RMGI. This study aimed to assess the effect of the addition of different amounts of 58S nano-BAG on FS and the antimicrobial activity of RMGI cement.
2. MATERIALS AND METHODS
2.1. Synthesis of 58S Nano-BAG
In this in vitro, experimental study, an organic matrix was prepared for the synthesis of 58S nano-BAG by the sol-gel technique, and SiO2:CaO:P2O5 with 60:36:4 weight ratio was synthesized. For this purpose, deionized water (118.70 cc) was mixed with 2 molars nitric acid (9.78 cc) and stirred for 15 min in a stirrer. An exothermic reaction was then started. Next, 108.30 cc of TEOS was added, and the mixture was stirred for another 30 min. Afterward, 11.01 cc of TEP was added and stirred for 20 min. In this phase, an acidic SOL was obtained, to which calcium nitrate tetra-hydrate was added and mixed for 45 min. Next, in order to obtain gel, the acquired sol phase was incubated for 7 days. The obtained gel was dried at 70°C for 72 h and then at 120°C for 72 h followed by 3 h of heating at 600°C and 2 h of heating at 700°C. The obtained powder was ground for 30 min in a satellite mill [16].
2.2. Fabrication of Specimens
The 58S nano-BAG was added to RMGI such that 58S BAG was weighed by a digital scale (Mettler Toledo, Columbus, Ohio, USA) and added to Fuji II LC RMGI cement (GC Corporation, Japan) in 10wt%, 20wt%, and 30wt% concentrations. They were well mixed with a plastic spatula. Empty amalgam capsules disinfected in Deconex for 3 days were used to mix the powder with dimethacrylate resin and polyacrylic acid liquid. In order to fabricate specimens for the disc diffusion test, the capsules were autoclave-sterilized and then the powder and liquid were mixed according to the manufacturer’s instructions (powder to liquid ratio of 3.2:1 g), placed in the capsules, and mixed in an amalgamator (Dentsply Caulk, York, PA, USA) for 10 s.
Molds measuring 2 x 2 x 25 mm were used for the fabrication of specimens for the FS test. The obtained paste was applied to the molds and condensed with a plastic spatula. A transparent Mylar strip was placed over the paste, and the length of the mold was hypothetically divided into 5 segments and light-cured five times by a LED curing unit (Woodpecker, Henan, China) with 600 mW/cm2 light intensity and 470 nm wavelength for 20 s using the overlapping technique (each part was light-cured for 40 s) [16]. Next, the bottom surface was light-cured for 100 s. The specimens were then gently removed from the molds and incubated at 37ºC and 100% humidity for one week.
For the fabrication of disc diffusion specimens, the paste was applied into a mold measuring 2 x 6 mm by a plastic spatula, condensed, and light-cured for 40 s from each side. It should be noted that the disc diffusion specimens were fabricated in a sterile environment under an airflow cabinet [17].
2.3. Study Groups
According to the Garcia article [15], in the One-Way ANOVA tab of the SPSS 11 software, considering α=0.05 and 80% power, the sample size of 10 was selected.
Four groups (n=10) were studied for the FS test as follows:
- Control group comprising of 100% RMGI powder
- 90% RMGI powder + 10% 58S nano-BAG
- 80% RMGI powder + 20% 58S nano-BAG
- 70% RMGI powder + 30% 58S nano-BAG
For the disc diffusion test, in addition to the abovementioned four groups, another control group containing 100% BAG was also considered.
2.4. FS Test
The FS test was performed using a universal testing machine (Zwick Roell, Germany). The specimens were placed in the machine and 2 N load was applied at a crosshead speed of 0.5 mm/min. Load application continued until the specimen was bent and then fractured [17]. The FS graph was also drawn on a computer monitor. The level of stress, strain, and maximum load applied to the objects were determined by the software (SPSS Inc, Chicago, Illinois, USA). The FS was then calculated using the formula below:
Where is the FS in MPa, P is the maximum load at the bending point in Newtons (N), L is the distance between the two arms in millimeters (mm), b is the width of the specimen in millimeters (mm), and d is the diameter of the specimen in millimeters (mm) [17].
Also, the modulus of elasticity was calculated based on the slope of the stress-strain graph using the formula [17] below:
2.5. Disc Diffusion Test
In the disc diffusion test, the diameter of the growth inhibition zone of Streptococcus mutans in blood agar plate was measured. The microorganisms were first pre-cultivated in brain heart infusion broth at 37°C for 18 h. After the formation of colonies, the microorganisms were swabbed on the agar plate using a sterile cotton applicator. Next, a prepared disc along with three antibiotic discs of amoxiclav, cephalexin, and cefixime were placed on each plate. The plates were then incubated at 37°C for 2 days, and the diameter of the growth inhibition zones formed around the discs was measured.
2.6. Statistical Analysis
Data were analyzed using SPSS version 11. Normal distribution of data was evaluated by the Kolmogorov-Smirnov test. The FS, modulus of elasticity (flexural modulus), and disc diffusion data were analyzed using one-way ANOVA. Wherever one-way ANOVA revealed a significant difference, pairwise comparisons were carried out using Tukey’s post-hoc HSD test. The significance level was considered 0.05.
3. RESULTS
Fig. (1) shows the size and shape of nano-BAG particles under a scanning electron microscope. The size of nano-BAG particles ranged from 79.90 to 147.50 nm. Fig. (2) shows the DTA of the nano-BAG powder at a heating rate of 5°C/min. Fig. (3) presents the X-ray diffraction (XRD) pattern of the nano-BAG powder. Fig. (4) shows the results of Fourier-transform infrared spectroscopy (FTIR) of the nano-BAG powder.
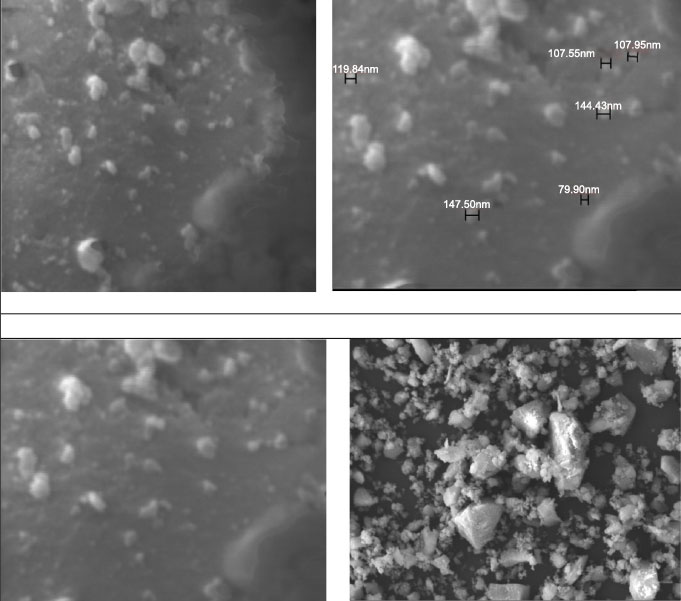
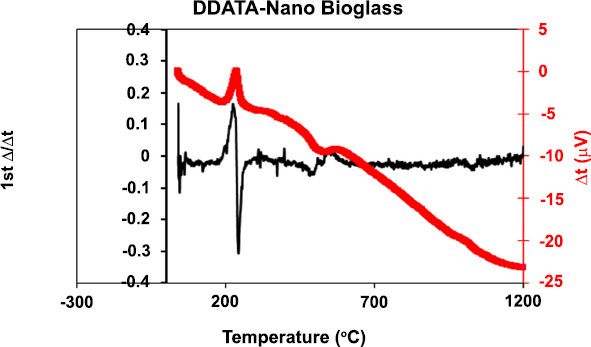
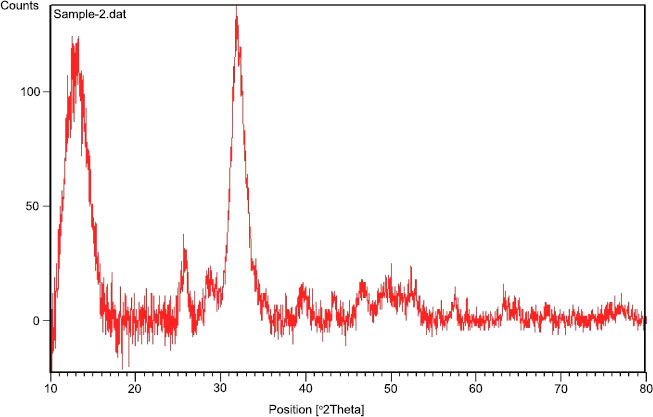
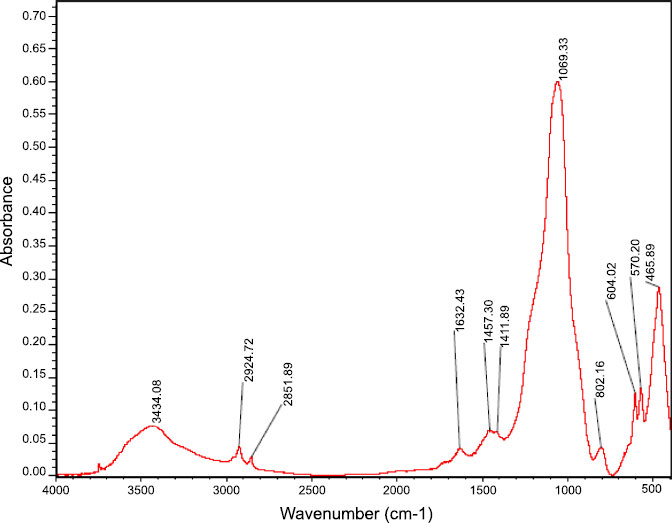
The Kolmogorov-Smirnov test confirmed the normal distribution of FS and disc diffusion test data in all groups. The raw data regarding the FS of 40 specimens (in 4 groups) and disc diffusion test results of 50 specimens (in 5 groups) are presented.
3.1. Flexural Strength (FS)
Table 1 and Fig. (5) present the mean and standard deviation of FS of the experimental groups. Statistical comparison of the groups with different weight percentages of BAG showed that increasing the percentage of 58S nano-BAG increased the FS of RMGI cement. However, one-way ANOVA revealed no significant difference in FS of the groups (P=0.06).
3.2. Antibacterial Activity
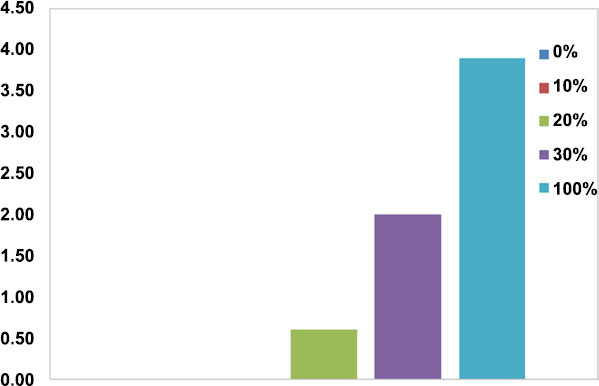
The results of the disc diffusion test revealed that the control group (100% nano-BAG) had the largest mean diameter of the growth inhibition zone while the minimum diameter of the growth inhibition zone was noted in the groups with 0% and 10wt% nano-BAG. Fig. (6) shows the antimicrobial properties of the tested materials. One-way ANOVA revealed a significant difference between the groups with different weight percentages of BAG regarding the mean diameter of the growth inhibition zone. The Tukey’s test showed that the groups containing 30wt% and 100wt% BAG were responsible for the significant difference in the results.
| 4 | 3 | 2 | 1 | Group |
|---|---|---|---|---|
| 54.71 | 45.62 | 43.61 | 38.71 | Mean |
| 14.25 | 15.89 | 17.34 | 8.81 | Std. deviation |

4. DISCUSSION
In this study, 58S nano-BAG powder was synthesized by the sol-gel technique. The TEOS, TEP, deionized water, and nitric acid were mixed and finally, the satellite mill was used to obtain particles in a nanometer scale. However, another study used a combination of ethanol with TEOS, deionized water, TEP, and nitric acid [18]. Chen et al. performed wet mechanical grinding in the final step to obtain BAG powder with nano-sized particles [19]. Hong et al. synthesized BAG nanoparticles via a two-step process of sol-gel and co-precipitation. They hydrolyzed TEOS, TEP, and calcium nitrate in an acidic environment, and condensed them separately in an alkaline state. Next, they performed freeze-drying. In this study, nitric acid was used to synthesize BAG [20].
However, Chen et al. used lactic acid to synthesize BAG by the sol-gel technique. They reported that addition of lactic acid decreased the size of nano-BAG particles. Moreover, it created porosities on the surface and increased its bioactivity compared with a smooth surface [21]. Chen et al. added ions such as zinc, magnesium, zirconium, titanium, silver, and boron to enhance the bioactivity and performance of BAG. However, addition of these ions complicated the synthesis of nano-BAG [22]. Thus, in this study, such ions were not added because decreasing the size of particles from micro-scale to nano-scale would per se improve the bioactivity. Catauro et al. prepared BAG by the sol-gel technique and added 100 nm silver nanoparticles to it. They reported that the addition of silver increased the number of Si-O-Si bonds and created a more compact structure with higher strength [23].
As shown in Fig. (1), the size of BAG particles ranged from 80-150 nm (mean of 115 nm). Such particles have a larger contact area and higher bioactivity than rougher crystals. This finding was in line with the results of previous studies [24, 25]. The presence of a sharp peak on the DTA graph (Diagram 1) indicates an exothermic reaction that occurs between 210-250°C. This exothermic reaction can be related to the release of gas components from the BAG during this process. This reaction may cause cracks in the drying phase in this temperature range. This finding was in agreement with that of Fathi et al. [26]. Diagram 2 shows the XRD graph. A sharp peak was noted at low temperature, which indicates crystalized structure. However, in the study by Fathi et al., no sharp peak was noted up to 900°C, and the BAG had an amorphous structure. However, crystallization occurred by increasing the temperature to 1000-1100°C. Formation of a crystalline phase decreases the bioactivity of BAG [26, 27]. Thus, the specimens should be heat-treated at a temperature lower than the crystallization temperature. In this study, after adding the desired weight percentage of nano-BAG, the powder and liquid were mixed according to the manufacturer’s instructions (3:2). Some studies have discussed that decreasing the powder to liquid ratio decreases the mechanical properties of glass ionomer cement [28, 29]; however, this is not true for RMGI [30]. Another study reported that the fracture toughness of RMGI was not highly influenced by the powder to liquid ratio. However, decreasing the powder to liquid ratio decreased the compressive strength of glass ionomer cement [31]. Mousavinasab et al. fabricated RMGI specimens according to the manufacturer’s instructions (powder to liquid ratio of 3:2) and used BAG in a 2:7 ratio to synthesize RMGI specimens containing 20wt% BAG. Yli-urpo et al. [32] fabricated RMGI specimens with a 3:2 powder to liquid ratio and used 2:5 and 2:7 ratios for the fabrication of specimens containing 10wt% and 30wt% BAG, respectively [33].
The agar disc diffusion test was used to determine the antimicrobial properties of RMGI containing BAG in this study. The growth inhibition zone was noted only around RMGI discs containing 20wt%, 30wt% and 100wt% BAG. The growth inhibition of S. mutans by RMGI cement may be due to low pH or the release of fluoride in an aqueous environment. However, the reason for growth inhibition in our study cannot be the low pH of the cement because the addition of BAG neutralizes the low pH of RMGI in an aqueous environment. Another study showed that glass ionomer discs containing 30wt% BAG released higher amounts of Ca, P, Si and F than glass ionomers without BAG. However, a glass ionomer containing 10wt% BAG released the same amount of fluoride [32]. Thus, the presence of the growth inhibition zone of Streptococcus mutans in this study was probably due to the increased release of fluoride ions.
Balamurugan et al. showed that bioactive gel glass with the composition of 64 SiO2, 26CaO and 10 P2O5 (in mol) had no antibacterial effect on Escherichia coli. The size of BAG particles in their study ranged from 100-700 μm. Thus, size of particles can increase the antibacterial activity of BAG containing high amounts of SiO2 [34]. Munukka et al. demonstrated that BAG produced by the gel-sol technique with high CaO content (42.3%) had greater antibacterial activity on aerobic bacteria than BAG with lower CaO concentration (31.27%) [35]. Also, Mortazavi et al. demonstrated that the broth containing 58S BAG had a higher pH than that containing 63S and 72S BAG, which is an indicator of higher antibacterial activity [36].
In fact, the basicity of a solution depends on the concentration of silica. The pH of a solution is influenced by the acid-base balance (including successful deprotonation and reprotonation) of (SiO4)-4, (HSiO4)3 and (HSIO4)-2 silica ions. The pH of a medium decreases as the SiO4 is solubilized. Next, CaO reacts with H2O and increases the pH of the medium. The 72S BAG has very high SiO2 content; thus, high amounts of silica are released into the broth and decrease the pH of the medium. This increases the rate of degradation and thus, higher amounts of Ca are released. The synergistic effects of high concentrations of calcium and the alkaline pH make 58S BAG a suitable antibacterial agent [37].
According to this study, 58S BAG had antimicrobial properties; this finding was in line with that of Munukka et al. [35]. On the other hand, the antimicrobial activity significantly increased by addition of 30wt% BAG to RMGI; however, addition of 10wt% and 20wt% caused no significant difference.
In this study, the three-point bending test was performed to determine the mechanical properties of RMGI containing different weight percentages of BAG. This test was used since dental materials are under flexural forces in the cervical region of the teeth. The FS of RMGI (Fuji II LC) was found to be 38.71±8.84 MPa in this study, which was almost equal to the FS values reported by Zhao and Zhang (35.8±4.1 MPa) [38]. However, this value was lower than the value reported by Mousavinasab et al. [39], (61.46±22.5 MPa) and Xie et al. [40], (52.8±1.9 MPa), which can be due to different conditions under which, the specimens were synthesized, or the conduction of tests. In this study, increasing the weight percentage of BAG added to RMGI increased the FS but not significantly. This result was not in accordance with the findings of Mousavinasab et al. [39]. In their study, addition of 20wt% BAG to RMGI significantly decreased the FS (39.90±9.1 versus 61.41±22.5 MPa). However, they reported that this value was still clinically acceptable. In their study, 45S5 BAG with micron-scale particles was used. Several studies have reported that the addition of 45S5 BAG with micron-scale particles to RMGI decreases the mechanical properties and increases the bioactivity [39]. However, in this study, increasing the weight percentage of BAG added to RMGI enhanced the antimicrobial property without decreasing the FS, which is clinically favorable. Recent research reported that remineralising molecules could be incorporated into composite resins [41]. Therefore, future research could test this important preventive feature also for glass ionomers.
Difference in the results of our study and those of previous studies may be due to the difference in the size of particles or type of BAG used since we used 58S BAG with nano-scale particles while the aforementioned studies used perioglass with micron-scale particles.
Decreasing the size of particles increases the surface area and subsequently the mechanical and biological properties [31, 37]. Another reason may be the application of 58S BAG in this study because this type of BAG contains a higher percentage of SiO2 (58%) than 45S5 BAG (45%). Increasing the concentration of SiO2 increases the contact area of particles and eventually the mechanical strength of specimens [40].
The FS of RMGI containing BAG in this study (43.6 to 54.7 MPa) was higher than the FS of glass ionomer and hydroxyapatite (GI-HA) in a previous study [40]. Thus, considering the role of mechanical properties and bioactivity in mineralization, the combination of RMGI and BAG seems to be more suitable than RMGI and hydroxyapatite.
Previous studies reported that the addition of 45S5 or 45S4 BAG with micron-size particles to glass ionomer decreased its mechanical properties and increased its bioactivity. Thus, its application was only suggested in non-stress-bearing areas in high-risk patients. However, considering the improved antimicrobial properties and no reduction in FS of RMGI in combination with BAG, increasing the weight percentage of BAG can further increase the applications of this compound.
There are some other promising materials like Phosphorene or Borophene that show different chemical and physical characteristics. They can be used as a biomaterial in dentistry. Thus, further experiments are needed to compare different biomaterials in order to choose the appropriate one in each situation [42, 43].
This in vitro study has some limitations with regard to clinical application. For example, the behavior of the materials in the oral environment may also change when exposed to saliva and continuous acidic challenges.
Therefore, it is suggested that some studies were conducted in conditions more similar to the real condition like the simulation of mechanical and physical properties of the oral environment and even performed it in-vivo.
CONCLUSION
Considering the study limitations, it seems that the addition of 30wt% nano-bioactive glass (BAG) to RMGI powder enhanced its antimicrobial activity without compromising its FS. Moreover, although the addition of 10wt%, 20wt% nano-BAG to RMGI powder did not significantly change the flexural strength; but had no significant effect on increasing antibacterial properties in comparison with RMGI without BAG. This conclusion is based on laboratory and in vitro studies. It should be carefully interpreted as the clinical performance of these materials needs further investigations and clinical trials.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [S.V.] on special request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


