All published articles of this journal are available on ScienceDirect.
A Case of Odontogenic Myxoma of the Mandible with Expansion to the Alveolar Crest – Comparison of Imaging Findings and Pathological Findings: A Case Report
Abstract
Background:
An odontogenic myxoma is an intraosseous tumor characterized by stellate and spindle-shaped cells embedded in an abundant myxoid or mucoid extracellular matrix. We herein describe an odontogenic myxoma that expanded not only to the bone marrow but also to the outside of the alveolar bone. Diagnosis of an odontogenic myxoma in a tooth-deficient region by imaging findings alone was difficult because the positional relationship between the tumor and the tooth is unknown. Furthermore, some of these odontogenic myxomas reportedly show rapid growth.
Case Report:
Here, we present the case of a patient, a 44-year-old man, who had a hard, bone-like swelling on his right mandible molar region and mild paresthesia on his right cheek. An odontogenic myxoma and ameloblastoma were suspected based on the imaging findings; however, pathological examination of the biopsy led to a diagnosis of odontogenic myxoma. Right segmental mandibulectomy was performed, and there was no recurrence observed after surgery.
Conclusion:
To improve the accuracy of imaging diagnosis, it is important to compare the imaging findings with the pathological findings of the surgical specimen. This comparison in the present case revealed differences in the magnetic resonance imaging signal intensity in regions with different types of cell components.
1. INTRODUCTION
An odontogenic myxoma is defined by the World Health Organization as an intraosseous tumor characterized by stellate and spindle-shaped cells embedded in an abundant myxoid or mucoid extracellular matrix [1]. Odontogenic myxomas reportedly account for 3% to 20% of all odontogenic tumors [2]. An odontogenic myxoma is the third most common odontogenic tumor (following odontoma and ameloblastoma) and most frequently occurs in the second to fourth decades of life [3-5]. Odontogenic myxomas are most commonly found in the molar region of the mandible [6, 7]. These tumors painlessly expand, and cortical perforation may occur when they become large. Radiographically, an odontogenic myxoma appears as a unilocular or multilocular radiolucency. The borders of the tumor are usually well-defined and corticated, and both root displacement and root resorption occur. Computed tomography (CT) may reveal fine bony septa and allow for anatomic delineation [8]. Gross examination reveals a grey-white mass with gelatinous contents. Histopathological findings are characterized by randomly oriented stellate, spindle-shaped and round cells. Odontogenic myxomas tend to permeate into marrow spaces, and larger lesions may require complete excision with tumor-free margins [9]. The average recurrence rate reported among previous studies is about 25%, but the prognosis is good [1].
We herein report a case of an odontogenic myxoma that expanded not only to the marrow space but also beyond the alveolar bone. Particular focus is placed on the radiologic-pathologic correlation of this odontogenic tumor.
2. CASE REPORT
In July 2019, a 44-year-old man visited a general dental practitioner with complaints of pain around the maxillary anterior teeth. A multilocular radiolucent lesion was observed in the right mandibular molar region on a panoramic image. The dental practitioner referred him to Okayama University Hospital in the same month for further examination.
On clinical examination at Okayama University Hospital, a hard, bone-like swelling was noted on his right mandible molar region, and mild paresthesia was present on his right cheek. Panoramic imaging showed a well-defined multilocular radiolucent lesion with bony septa and an expanded cortex in the right mandibular molar region (Fig. 1). On CT, the axial image of the bone showed an expanded cortex and bony septa (so-called tennis racket-like septal structure) in the right mandibular molar region (Fig. 2a). The sagittal image of the bone showed cortex expansion and mandibular canal deviation (Fig. 2b). The contrast-enhanced axial image of the soft tissue showed low enhancement (Fig. 2c). On magnetic resonance imaging (MRI), axial T1-weighted imaging (T1WI) showed homogeneous isointensity (Fig. 3a). Axial short T1 inversion recovery (STIR) imaging showed relative hyperintensity to marked hyperintensity (Fig. 3b). On contrast-enhanced MRI (CE-MRI), axial contrast-enhanced T1WI showed faint enhancement except in the center of the lesion (Fig. 3c).
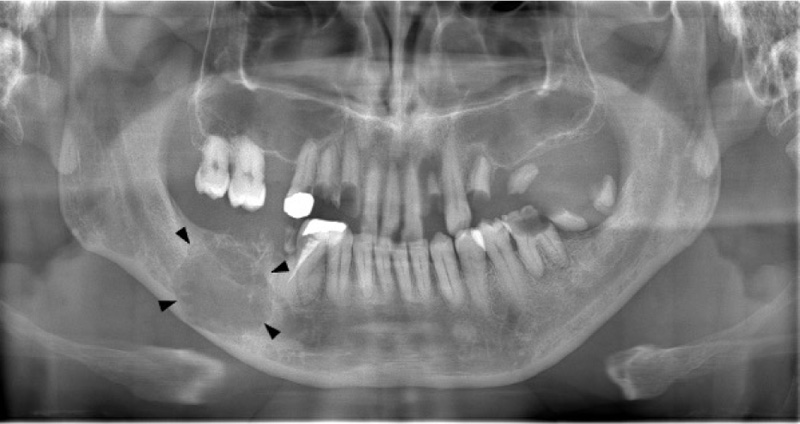
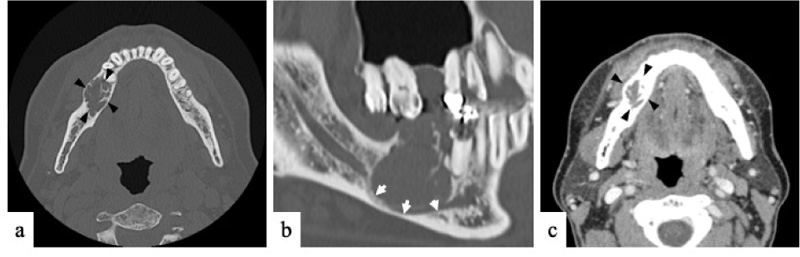
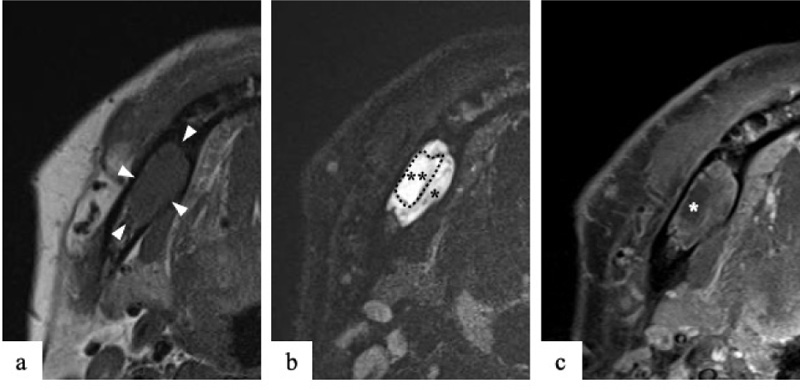
The tennis racket-like septal structure was found on CT images of the bone window setting. However, some parts of the lesion were enhanced on both CE-CT and CE-MRI. An odontogenic myxoma as well as ameloblastoma were suspected based on the imaging findings.
A biopsy was performed to obtain a definitive diagnosis. The lesion was white and fragile and contained mucoid material. Pathological examination of the biopsy specimen led to a diagnosis of odontogenic myxoma. A right segmental mandibulectomy was performed. After resection, the plate was fixed to the bone defect with screws.
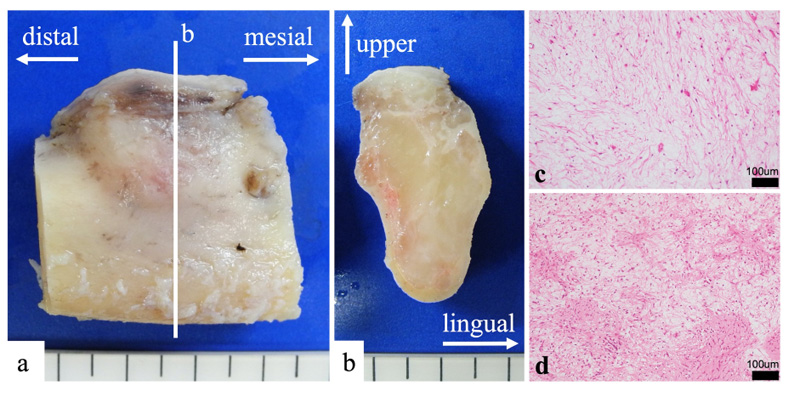
The mandibular specimen was sliced parallel to the tooth axis (Fig. 4a). The lesion was solid and milky white (Fig. 4b). It had no capsule and showed unclear boundaries. Moreover, it had expanded, causing thinning of the cortex bone. Buccal side of the lesion had abundant mucus (Fig. 4c), and the lingual side had abundant fiber (Fig. 4d).
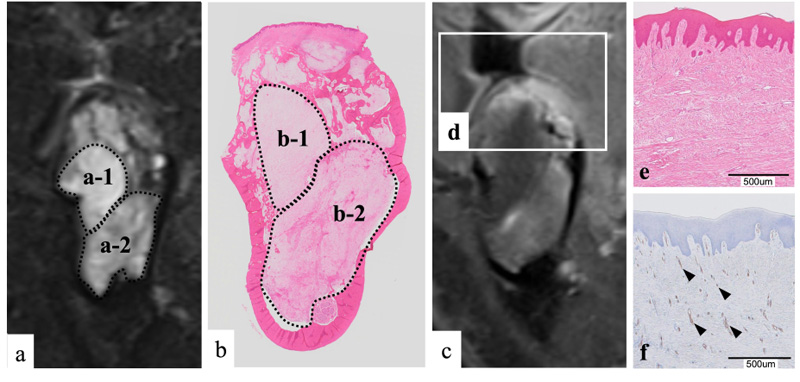
We compared the coronal STIR imaging findings and the pathological findings (Fig. 5a, b). In the loupe image of the pathological specimen, the lesion had expanded and destroyed the bone of the alveolar apex side (Fig. 5b). The magnified image showed proliferation of spindle-shaped cells, and mucus components were abundant among the cells (Fig. 5a). The cells showed a minor degree of abnormal size variation and had poor mitotic figures. There were no findings suggesting malignancy. Fibers were rich in some parts of the lesion (Fig. 5a). Therefore, the pathological diagnosis was an odontogenic myxoma.
3. DISCUSSION
This case involved an odontogenic myxoma in a lost tooth region. Six cases of odontogenic myxomas occurring in tooth-deficient regions have been reported to date [10-15]. In each case, it was difficult to distinguish the odontogenic myxoma from other odontogenic tumors by imaging findings alone because the positional relationship between the tumor and the teeth was unclear. Furthermore, some odontogenic myxomas that develop in tooth-deficient regions reportedly show rapid growth [11]. In cases showing rapid growth, the clinician may easily suspect a malignant tumor or non-odontogenic tumor that shows a strong proliferative tendency. A biopsy should be performed to avoid misdiagnosis [16, 17]. Furthermore, to improve the accuracy of the imaging diagnosis, it is important to compare the imaging findings with the pathological findings of the surgical specimen. Such comparison in the present case showed that the region exhibiting hyperintensity on STIR imaging was rich in mucus components (Fig. 5a-1, b-1). In contrast, the region showing relative hyperintensity on STIR imaging was rich in fibers (Fig. 5a-2, b-2). Moreover, the alveolar apex showed hyperintensity on contrast-enhanced T1WI (Fig. 5d), and in hematoxylin-eosin staining, the subepithelial connective tissue was strongly eosin-stained (pink); this staining tendency indicated fiber proliferation (Fig. 5e). Immunohistochemical staining using CD34 as a general blood vessel marker showed strong CD34 positivity in the subepithelial connective tissue, indicating the proliferation of micro-blood vessels (Fig. 5f).
Therefore, there was no significant discrepancy observed between the imaging findings and pathological findings in this case. Regions of odontogenic myxoma rich in fibers reportedly show low contrast [18-20], and this tendency should be emphasized when making a diagnosis. An odontogenic myxoma with abundant fibers was once classified as an odontogenic myxofibroma; however, according to the 2017 World Health Organization classification, odontogenic myxofibromas are now integrated into odontogenic myxomas [1].
In the present case, the tumor showed high contrast when it extended outside the bone, and the blood vessels and fibers grew reactively. In such cases, it becomes difficult to rule out an epithelial tumor with contrast-enhanced examinations, such as CE-CT and CE-MRI. Therefore, the findings both outside and inside the bone are important for imaging diagnosis. An odontogenic myxoma is a mesenchymal tumor consisting of stromal components with low intercellular connectivity, unlike epithelial tumors in which epithelial cells with intercellular connectivity mainly proliferate. Thus, odontogenic myxoma cells are considered to proliferate until they penetrate the trabecular bone without distorting the running course of the existing trabecular bone, and the remaining relatively thick trabecular bone exhibits a tennis racket-like structure. However, no reports have described the pathological mechanism underlying the formation of the characteristic trabecular pattern found in odontogenic myxomas. Further pathological studies are needed to elucidate the mechanism of the characteristic bone resorption pattern caused by odontogenic myxoma.
CONCLUSION
We have herein described a case of an odontogenic myxoma that spread significantly beyond the alveolar bone and compared the imaging findings with the pathological findings to achieve a diagnosis. No significant difference was found between the imaging and pathological findings. A biopsy should be performed to avoid misdiagnosis when similar cases are encountered. Moreover, to improve the accuracy of imaging diagnosis, the pathological findings should be confirmed post-operatively.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This case report was approved by the Okayama University Ethics Committee (No. 2012-010).
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. All procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
CONSENT FOR PUBLICATION
Informed consent was obtained from the patient for being included in the study. The patient provided permission for his information to be published.
STANDARDS OF REPORTING
CARE guidelines and methodologies were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
None.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors thank Angela Morben, DVM, ELS, from Edanz Group (https://en-author-services.edanz.com/ac), for editing the draft of this manuscript.


