All published articles of this journal are available on ScienceDirect.
Analysis of Expression of Myofibroblasts in Oral Submucous Fibrosis: An Immunohistochemistry Study
Abstract
Background:
Oral submucous fibrosis (OSMF) is a chronic disease that produces tissue fibrosis and is considered to be a potentially malignant disorder. The exact pathogenesis and malignant conversion mechanism of this disorder are still unknown. Myofibroblasts have been implicated as one of the possible pathological mechanisms responsible for the pathophysiology of OSMF. The present study was conducted to evaluate the expression of myofibroblasts (MF) in normal mucosa and different grades of OSMF.
Materials & Methods:
The sample consisted of a total of 80 specimens. The study group included specimens from clinically and histopathologically confirmed OSMF patients. The specimens were divided into four groups. Group 1 consisted of 19 specimens of grade III OSMF. Group II had 20 specimens of grade II OSMF, Group 3 with 21 specimens of grade I OSMF, and Group 4 constituted a control group of 20 normal epithelium specimens. Two sections each from all the four groups were obtained. While one section was stained with H and E, the other section was stained immunohistochemically using α-smooth muscle antibody. For analysis, the expression of myofibroblasts was categorized as strong, moderate, weak, or absent. All the results were recorded and subjected to statistical analysis.
Results:
In OSMF patients, irrespective of the grade, the expression of myofibroblast was strong in 28.33 percent of the patients, while it was moderate and weak in 30.00 percent and 40.00 percent of the patients, respectively. Expression of myofibroblast was noted to be significantly increased in grade III OSMF patients as compared to controls as well as grade I OSMF patients (p-value <0.05).
Conclusion:
Myofibroblasts expression is significantly raised in OSMF patients. The expression can also be correlated within different grades of OSMF where advanced stages show comparatively high expression of these smooth muscles like fibroblasts. Hence, we suggest that myofibroblasts could be assessed as markers for analyzing the progression of OSMF.
1. INTRODUCTION
Oral submucous fibrosis (OSMF) is a chronic progressive disease that produces scars and tissue fibrosis; it is considered to be potentially malignant.
Epidemiological studies have revealed that cases of OSMF occur globally. In India, most of the cases are concentrated in South India, and the incidence and prevalence of OSMF have increased manifolds from an estimated 250,000 cases in 1980 to 14 million cases in 2010 [1]. Pathological characteristics include chronic inflammation in lamina propria or deep connective tissues with collagen deposition below the oral mucosal epithelium along with degenerative changes in the muscles [2]. Various hypotheses proposed in the past suggest a multifactorial origin for this condition ranging from areca nuts, spices, tobacco, as well as genetic propensity [3].
The exact pathophysiological and molecular mechanism of development of OSMF and its advancement to malignancy is still unclear. The emerging pattern suggested is the injury leading to inflammatory response and release of mediators that cause epithelial-mesenchymal transition (EMT), causing fibrosis. The roles of extracellular matrix (ECM) formation and EMT in OSMF are supported by the fact that many mediators such as cytokines and proteins and many signaling pathways involved had been activated in disorders like OSMF, which alter the normal healing process [4].
The most common signs and symptoms include dry mouth, blanched leathery mucosa, burning sensation, pain, restricted tongue mobility, depapillation, trismus, and dysphagia [5]. OSMF contributes significantly to mortality because of its high malignant transformation rate that may range from 1.5-15% [6].
The myofibroblasts can be stated as simply smooth muscle-like fibroblasts. They are addressed as either activated smooth muscle cells or lipocytes as they have a propensity to store retinoids and can be addressed as stellate cells that change shape during transient differentiation [7]. Whenever a cell is injured, the fibroblasts are recruited locally, possibly originating myofibroblasts. The vascular smooth muscle cells around the vessels and epithelial-mesenchymal transition could be another possible source of origin of myofibroblasts [8].
Myofibroblasts have been implicated as one of the possible pathological mechanisms responsible for OSMF and its conversion to malignancy; thus, they need to be studied extensively in these disorders. The present study was conducted to evaluate the expression of myofibroblasts in different grades of OSMF, considering the hypothesis that myofibroblasts play a role in the advancement of OSMF and may be one of the factors responsible for malignant transformation.
2. MATERIALS AND METHODS
The present cross-sectional in vitro study was conducted to evaluate the expression of myofibroblasts in OSMF patients. The patients reported to the OPD of a reputed dental college in the north who were clinically and histopathologically diagnosed with OSMF were included in the study. OSMF was categorized according to the grading given by Passi D et al. in 2017 [9]. The ethical committee clearance was taken from the institutional ethical committee with the ethical number ITS/02/2056/2020. All the subjects were briefed about the study, and written informed consent was obtained.
For this immunohistochemical study, all the samples obtained were freshly stained, and formalin-fixed and paraffin-embedded tissue blocks were constructed. The specimens obtained from the study patients were divided into four groups. Group 1 consisted of 19 specimens of grade III OSMF, Group II with 20 specimens of grade II OSMF and Group 3 with 21 specimens of grade I OSMF. Group 4 constituted a control group of 20 normal epithelium specimens taken from the patients who were undergoing minor oral surgical procedures such as frenectomies, extractions, and flap surgeries in the dental hospital.
Two sections each from both the OSMF patients and the control group were obtained. One section was stained with H and E, and findings were recorded, while the other section was stained immunohistochemically using α-SMA antibody by indirect peroxidase-antiperoxidase. The kit used was provided by Leica Biosystems, New Delhi. The standard procedure of IHC was used for performing analysis. In order to evaluate the specificity of the immunoreactions, both known positive and negative tissue controls were considered. The fibroblasts in the control group simulated the positive control, and the omission of antibodies was taken as the negative control.
The handling of tissue for IHC is technique sensitive; therefore, for the adhesion of the tissues for the IHC procedure, the slides were coated with the poly-L-lysine solution. Fixation of the tissues was done in 10% formalin, and thin 3-5-micron sections were taken to assess the results.
Stromal spindle cells positive for α-SMA were considered as myofibroblasts. The immunostaining for α-SMA was carried out, involving the demonstration of antigens in tissues and cells by the binding of an antibody to the antigen of interest. This step was followed by the detection of the bound antibody by an enzyme chromogenic system.
Two experienced oral pathologists with an average of 5 years of experience interpreted the samples in all four groups. All the sections were counted twice to avoid intra-observer variability. The samples with a hundred percent inter-observer agreement were included in the study and analyzed statistically to remove any bias from inter-observer variability. Both the oral pathologists were completely blinded about the samples while reporting.
The evaluation was done by assessing the three parameters: percentage of staining positive cells for alpha-SMA, staining intensity, and finally, the staining index.
Staining intensity was evaluated as follows: 0% = with no staining; 1% = staining positivity observed only at a magnification of ×400; 2% = staining was obvious at ×100 only, and 3% = where positivity was appreciable even at ×40.
For staining percentage, the percentage of cells was calculated. No positive cells were considered as 0, 1-25% of positive cells were given a score of 1, 25-50% positive cells were considered as score 2, and >50 positive cells were given a score of 3.
Calculation of the staining index was done by multiplying staining intensity and percentage of staining cells. The final expression of myofibroblasts or staining index was interpreted as strong (score above 4), moderate (score 3-4), weak (score 1-2), or absent (score 0).
All the results were recorded and analyzed by SPSS software version 23 for statistical analysis.
| Expression of Myofibroblast |
Group 1 N= 19 Grade III |
Group 2 N= 20 Grade I |
Group 3 N=21 Grade I |
Control Group (n=20) | Percentage Expression of Staining Index |
| Strong | 9 | 5 | 3 | 0 | 28.33% |
| Moderate | 6 | 7 | 5 | 0 | 30% |
| Weak | 4 | 8 | 12 | 0 | 40% |
| Absent | 0 | 0 | 1 | 20 | 1.66% |
3. RESULTS
Table 1 represents the mean and standard deviation of staining intensity, staining percentage, and staining index in the control group and various grades of OSMF.
Table 2 represents the number of samples showing the final expression of myofibroblast or staining index in control and study groups. Expression of myofibroblast was noted to be negative in all the patients of the control group (Group 4). In the OSMF group (Grade I, Grade II, and Grade III combined), expression of myofibroblast was strong in 28.33 percent of the patients, while it was moderate and weak in 30 and 40 percent of the patients, respectively.
In Table 3, the Fisher Halton Exact Test was applied to compare the expression of the percentage of the myofibroblasts in different groups. The p-value was found to be highly significant (p=0.000) when OSMF patients, irrespective of grades, were compared with the control group. The results revealed that myofibroblasts were significantly increased in OSMF patients.
| - | No. of Samples | Staining Intensity | Staining Percentage | Staining Index | |||
| Grade III OSMF | 19 | 2.50 | 0.78 | 1.90 | 0.83 | 4.75 | 0.40 |
| Grade II OSMF | 20 | 1.98 | 0.80 | 0.73 | 0.98 | 1.94 | 0.80 |
| Grade I OSMF | 21 | 1.20 | 0.70 | 0.81 | 0.46 | 0.97 | 0.50 |
| Control group | 20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Groups |
Absent N(%) |
Weak N(%) |
Moderate N(%) |
Strong N(%) |
Total N(%) |
Fisher Halton Exact Test | |
| p-value | Significance | ||||||
|
Grade III OSMF Group 1 |
00 (0.0) | 04(21.1) | 06(31.6) | 09(47.4) | 19(100.0) | 0.000 | Significant |
|
Grade II OSMF Group 2 |
00 (0.0) | 08(40.0) | 07(35.0) | 05(25.0) | 20(100.0) | ||
|
Grade I OSMF Group 3 |
01(4.8) | 12(57.1) | 05(23.8) | 03(14.3) | 21(100.0) | ||
|
Control |
20 (100.0) | 00(0.0) | 00(0.0) | 00(0.0) | 20(100.0) | ||
| Groups |
Absent N(%) |
Weak N(%) |
Moderate N(%) |
Strong N(%) |
Total N(%) |
Chi-Square/Fisher Halton Exact Test | |
| p-value | Significance | ||||||
|
Grade I OSMF Vs Grade II OSMF |
01(4.8) | 12(57.1) | 05(23.8) | 03(14.3) | 21(100.0) | 0.456 | Non-Significant |
| 00 (0.0) | 08(40.0) | 07(35.0) | 05(25.0) | 20(100.0) | |||
|
Grade II OSMF Vs Grade III OSMF |
00 (0.0) | 08(40.0) | 07(35.0) | 05(25.0) | 20(100.0) | 0.282 | Non-Significant |
| 00 (0.0) | 04(21.1) | 06(31.6) | 09(47.4) | 19(100.0) | |||
|
Grade I OSMF Vs Grade III OSMF |
01(4.8) | 12(57.1) | 05(23.8) | 03(14.3) | 21(100.0) | 0.046 | Significant |
| 00 (0.0) | 04(21.1) | 06(31.6) | 09(47.4) | 19(100.0) | |||
Table 4 depicts the comparison within different grades of OSMF. No significant difference in the myofibroblasts was seen in the comparison of grade 1 and grade 2 OSMF and also while comparing grade III and grade II OSFM. The results were statistically significant when comparing grade I and grade III subjects, with a p-value of 0.005. Thus, the maximum expression of myofibroblasts was found in grade III OSMF patients.
Fig. (1) shows normal epithelium, Fig. (2) shows myofibroblasts expression in grade II OSMF, and Fig. (3) represents the expression of myofibroblasts in grade III OSMF.
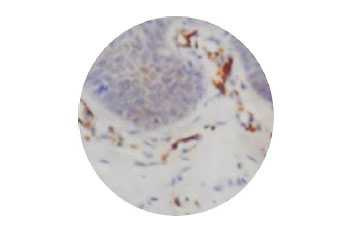

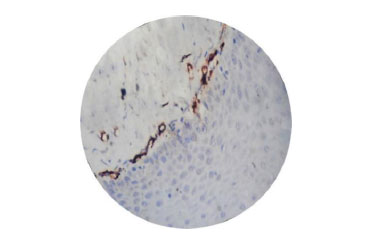
4. DISCUSSION
Oral submucous fibrosis (OSMF) is a premalignant disorder mainly associated with the chewing of areca nut (betel nut). The habit is more prevalent in South Asian populations but has been increasing in Europe and North American regions. The increase in consumption of areca nut and tobacco, along with their easy availability, has led to a sharp rise in the incidence and prevalence of OSMF.
OSMF has been considered under the classification of potentially malignant disorders by the WHO Collaborating Centre for Oral Cancer and Precancer since 2008 [10]. It has a high malignant transformation rate, but the reason behind the mechanism of malignant conversion is still controversial and unclear.
Various pathological mechanisms have been described for OSMF, but still, the exact pathogenesis remains unclear. By considering the role of myofibroblasts in the pathogenesis and malignant transformation of this morbid premalignant disorder, the present study was planned to evaluate the expression of myofibroblasts in different grades of OSMF.
Myofibroblasts have been identified in most fibrotic conditions. Myofibroblasts are unique cells resembling smooth muscle cells and fibroblasts and can be located by the expression of alpha-smooth muscle actin (α SMA). Newer studies on pathophysiology suggest the possible role of extracellular matrix (ECM) and epithelial-mesenchymal transition (EMT) in the advancement of this disorder as well as conversion to malignancy [11].
Fibrosis is usually a part of the wound-healing mechanism when in check but could be pathogenic if it remains uncontrolled, leading to scarring by remodeling of extracellular matrix (ECM) [12]. Recent evidence appreciates the belief that conversion of fibroblast to myofibroblasts is important for the cells to perform healing functions. It is believed that myofibroblasts play a primary role in the production of ECM after injury and thus can be related to the possible pathogenesis of OSMF [13].
In the present study, expression of myofibroblast was found to be negative in all the normal epithelium specimens (control group). In the OSMF patients, irrespective of the grade, the expression of myofibroblast was strong in 28.33 percent of the patients and weak in 40 percent of the patients.
Angadi et al. studied 70 OSMF patients, noted the increased presence of myofibroblasts in OSMF, and suggested that OSMF is an abnormal healing process in response to chronic irritation. Their potential use as markers for evaluating the severity of OSMF has also been suggested [13].
Our results were found to be consistent with the results of Philip et al. (2014), who also found a significant increase in the staining index of myofibroblasts in different histological grades of OSMF [1].
Gandhi P et al. compared the presence of myofibroblasts in normal mucosa, different grades of OSMF, and oral squamous cell carcinoma (OSCC). The results of this study were in accordance with the present study, where the presence of myofibroblasts was found to be significantly higher in OSMF cases when compared with normal epithelium specimens. The presence of myofibroblasts was also significantly higher in OSCC cases. These findings favored the possibility that OSMF could represent an abnormal healing process in response to irritational factors such as areca nut and other etiological agents [14].
The presence of myofibroblasts has been described and studied in most fibrotic conditions characterized by tissue retraction and remodeling [15]. In the present study, the expression of myofibroblast was significantly increased in OSMF patients compared to normal epithelium.
Filament systems of human cells, actin, vimentin, desmin, laminin, or glial fibrillary acidic proteins differentiate myofibroblasts from smooth muscle cells. Beta and gamma actins are expressed by all cells, including myofibroblasts that stain positively for α smooth muscle actin (SMA) [7]. In addition to α SMA expression, few newer biomarkers for myofibroblasts have been studied, such as soothelin and xylosyl transferase-I activity as proposed by various authors [16, 17].
Gupta K et al. compared the distribution of myofibroblasts using alpha-smooth muscle actin (α-SMA) in oral leukoplakia, OSMF, and various histopathological grades OSCC. Sixty formalin-fixed paraffin-embedded tissue blocks consisting of leukoplakia, various grades of dysplasia, OSMF, and OSCC were subjected to immunohistochemistry. On comparison of the frequency of mean scores in OL, OSMF, and OSCC, the values were 0.6 ± 0.2 (0-2), 1.2 ± 0.68 (1-2), and 2.6 ± 1.34 (0-4), respectively, which is in line with the results of the present study in the OSMF group. The authors concluded that MFs create a permissive environment for tumor invasion in OSCC and also have a role in the pathogenesis of OSMF [18].
Immunohistochemistry (IHC) is a promising adjunctive technique in the evaluation of soft tissue pathologies. Many pathologies, mainly fibrous, fibro-histiocytic, and lipomas, are still based on histology, but few still remain unclassified due to variability and complexity in the expression of many antigens. Many antibodies were considered to be effective only in frozen sections, but in IHC, newer modalities of antigen recovery and retrieval have made possible the application of many antibodies, thus accentuating the diagnosis [19].
OSMF shows a high malignant conversion rate as reported in the literature. Thus, the present study evaluated the expression of myofibroblasts in different grades of OSMF. There was no statistical difference obtained on comparing grade 1 and grade II specimens, which could be attributed to a bit smaller sample size for each grade. However, there was a highly significant increase in MF expression when grade I and grade III were compared, giving a possibility that the expression of these smooth muscle cells could be related to the severity of the disorder and may have a potential role in the malignant conversion of OSMF.
CONCLUSION
Expression of myofibroblasts is significantly raised in OSMF patients compared to healthy controls. As the grade of OSMF advances, the expression of myofibroblasts also increases. Hence, we suggest that myofibroblasts could be assessed as markers for analyzing the progression of OSMF. Future studies are required to study the exact role of myofibroblast expression in the malignant transformation of OSMF.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The ethical committee clearance was taken from the institutional ethical committee with ethical no of ITS/02/2056/2020.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written consent was obtained from the patients.
STANDARDS OF REPORTING
STROBE guidelines and methodologies were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


