All published articles of this journal are available on ScienceDirect.
Gingival Squamous Cell Carcinoma Masquerading as Localized Periodontal Disease in the Maxilla: A Case Report
Abstract
Objectives:
Squamous cell carcinoma is a malignant neoplasm of epithelium. In the U.S., carcinoma of the gingiva constitutes 4% to 16% of all oral carcinomas. This case report highlights such a case in maxillary gingiva and emphasizes the vital role of dental professionals, especially periodontists and endodontists, in being cognizant that an inflammatory lesion can mimic a serious condition like squamous cell carcinoma.
Case Presentation:
A patient who visited the screening clinic for an ulcerated lesion in the gingiva was otherwise healthy with no associated history of tobacco or any traumatic/persistent traumatic events.
The patient was treated for pseudoepitheliomatous hyperplasia based on the current condition and negative history of malignancy. On three months of follow-up, computed tomography radiographic evaluation and a biopsy were done, which were positive for the malignancy. Follow-up after six months includes PET, Head MRI, and chest X-ray examinations to rule out any metastatic entity.
Results:
A six-month follow-up radiographic examination revealed metastasis of the entity. Additional findings include pleural effusion and underlying infections.
Conclusion:
Early diagnosis is the key to the treatment plan. It further highlights the role of advanced imaging as a vital tool in determining the extent of the disease. Therefore, any persistent lesion exhibiting features that are not responding to conventional gingival and periodontal treatment options for more than two weeks should be referred for further evaluation to rule out cancer.
1. INTRODUCTION
Amongst the wide spectrum of pathological conditions encountered in the oral cavity, several conditions mimic each other and put the clinician at a diagnostic crossroad that can be very daunting. This is particularly true when the treatment is significantly different for each of the conditions on the differential diagnosis list. One such challenging conundrum is a similarity in the presentation of some inflammatory conditions that can mimic malignant conditions and vice versa. Several times gingival squamous cell carcinoma can mimic localized periodontal disease and is often misdiagnosed and mismanaged. The diagnosis of gingival squamous cell carcinoma (SCC) is critical because of the time-sensitive nature of the condition. Accurate diagnosis is vital as tumor size and invasion into the surrounding anatomical structures and timely intervention decides the prognosis [1, 2].
Gingival squamous cell carcinoma often presents with benign features, and if misdiagnosed or not diagnosed in the early stages, it can lead to a delay in executing the appropriate treatment. Gingival squamous cell carcinoma is often asymptomatic, and initial symptoms are usually an intraoral mass or swelling, ulceration, pain, ill-fitting dentures, mobility of teeth, or unhealed extraction wounds [3-5]. Computerized tomography (CT) and magnetic resonance imaging (MRI) are useful for evaluating the extent of carcinoma in the head and neck. This study highlights the clinical-histopathological and radiographic findings of a case of gingival SCC.
2. CASE DESCRIPTION
2.1. Patient Information and Relevant History
A 50-year-old male patient presented to a private dental office with the chief complaint of a lesion in the gums below heavily restored right maxillary first molar and second premolar. The patient was otherwise healthy with no history of tobacco use or any traumatic event.
2.2. Clinical Findings, Radiographic Findings, and Management
On clinical examination, an ulcerated lesion in the gingiva was present in the right posterior maxillary region (Fig. 1). Periapical and panoramic radiographs were done to evaluate the lesion further. Radiographic examinations revealed alveolar bone loss around the right maxillary first molar and second premolar and the canine, but the generalized alveolar bone loss was noted, with several other teeth in the oral cavity. Change in the trabecular pattern was observed in this area but was deemed to be the result of inflammation and possible infection (Fig. 2). Based on these findings, it was diagnosed as localized chronic severe periodontitis.
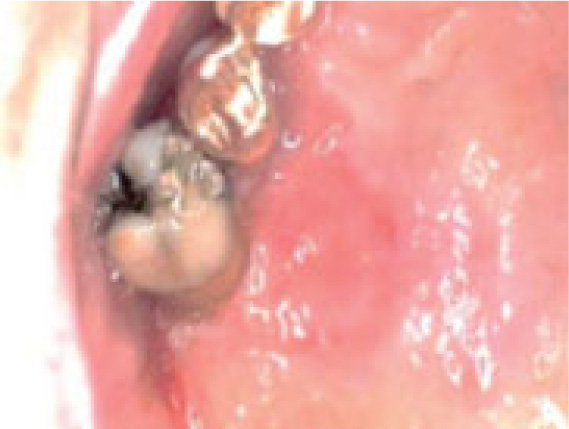
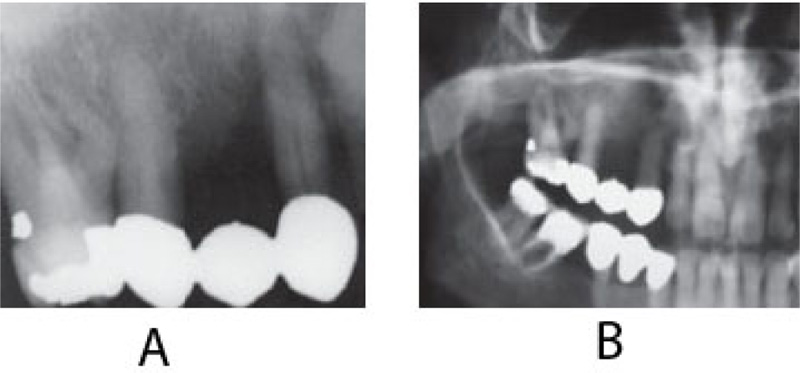
With the right maxillary first molar showing prognosis, the tooth was extracted. At a follow-up visit after three months, the lesion did not resolve after dental extraction and antibiotic therapy. Moreover, three months post-extraction, the mass showed rolled and raised borders (Fig. 3).

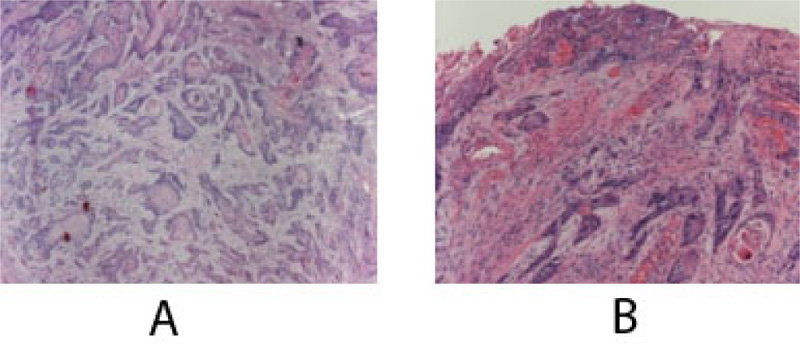
At this time, the case was referred to the University Hospital, and a computerized tomography scan (CT) was done to evaluate the lesion further. CT scan revealed an enhancing soft tissue mass in the right gingiva destroying the palate and causing irregular thinning of maxilla and adjacent maxillary sinus wall. The scan also showed enlargement of the lymph nodes (Fig. 4). Subsequent histology confirmed the diagnosis of a well-differentiated squamous cell carcinoma SCC (Fig. 5).


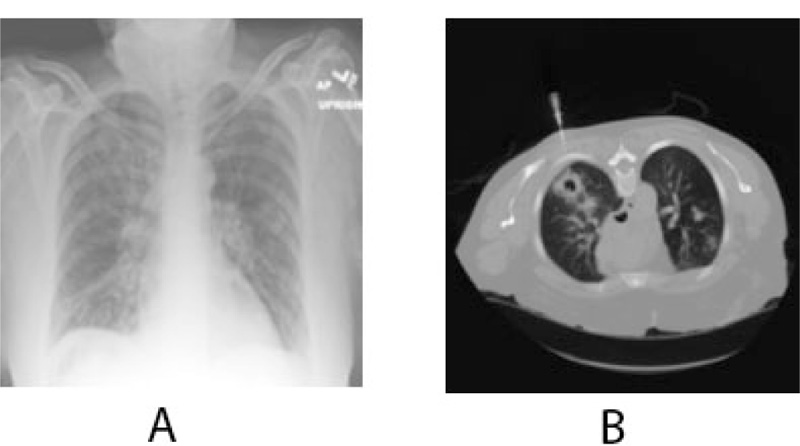
The patient eventually underwent multiple resections of the maxilla at the initial site of the mass and multiple neck dissection surgeries for the next six months. The biopsy results repeatedly revealed metastatic disease spreading to the lower neck lymph nodes. Positron Emission Tomography (PET/CT) scan result showed increased metabolic activity posterior to the sternocleidomastoid, contralateral neck, and within the lung parenchyma (Fig. 6). Head MRI was performed to detect metastatic spread to the brain and maxillofacial regions and showed no focal mass, abnormal enhancement, midline shift, or acute intracranial hemorrhage. A chest x-ray confirmed metastasis to the lungs (Fig. 7). To this date, the patient’s condition is worsening with progressive metastasis, pleural effusion, and underlying infections.
3. DISCUSSION
Gingival squamous cell carcinoma accounts for less than 10% of all oral cancers [6]. It is mainly reported in the elderly, with about 2% of the patient population reporting less than 40 years of age [7]. Gingival carcinomas are usually squamous cell carcinomas that are typically well-differentiated [8]. It is predominant in males. Carcinoma of the gingiva is insidious in growth and painless. One common finding is that in its early stages, it is often misdiagnosed as inflammatory lesions of the periodontium, such as pyogenic granuloma, periodontitis, papilloma, or even fibroid epulis (inflammatory hyperplasia) [8]. As in our study, the lesion mimicked localized chronic severe periodontitis and possibly a pseudoepitheliomathous hyperplasia.
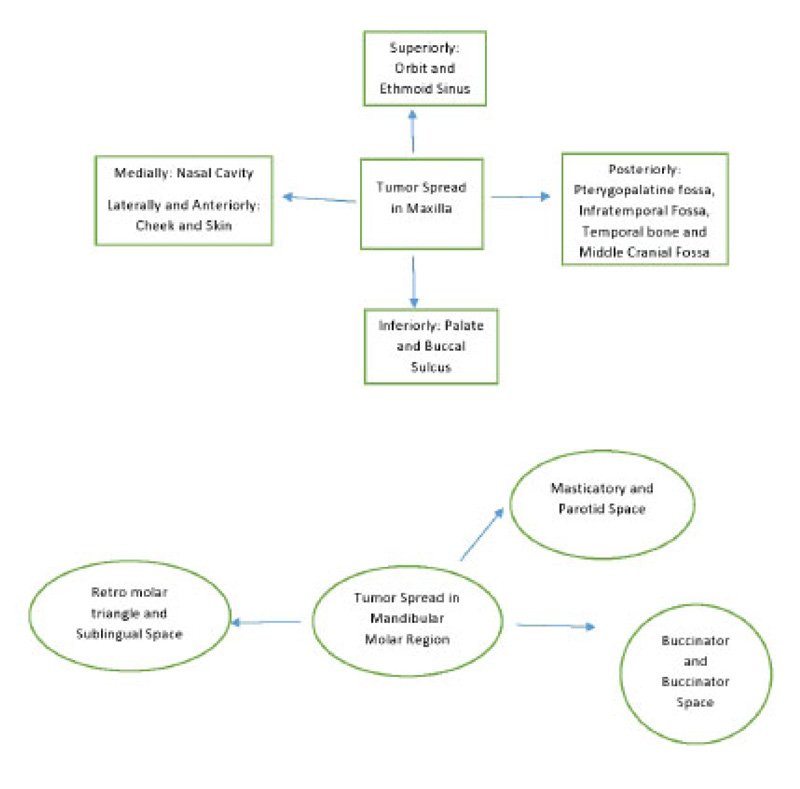
Most gingival squamous cell carcinomas are found in the mandible. Interestingly, in this case, it was in the maxilla. The site of the mass is very important as it is vital for the identification of the spaces and location of tumor spread. A schematic diagram of the tumor spread in the maxilla and mandible is described in Fig. (8). Cady et al., in 1969, did a 20-year survey on epidermoid carcinoma of the gum and found that posterior lesions tend to be larger with involvement of bone and other adjacent structures [9]. In this case, too, the initial lesion was in the posterior maxilla. This suggests the pivotal role of advanced imaging inappropriate diagnosis, delineating the extent and possible spread of the disease.
While evaluating the extent, the buccal and gingival areas of the lesion should be carefully evaluated for the extent of submucosal spread, osseous involvement, involvement of the retromolar trigone, pterygomandibular raphe, and cervical lymphatic spread [10]. The presence of osseous involvement is suggestive of a T4 lesion. The staging of the disease is important both for the treatment and prognosis.
Computed tomography (CT) is excellent at showing the subtle cortical erosions, and the extent of marrow involvement may be better assessed by using magnetic resonance (MR) imaging. CT findings of osseous involvement include cortical erosion surrounding the primary lesion, aggressive periosteal reaction, abnormal attenuation in bone marrow, and pathologic fractures [11]. In this case presentation, CT findings revealed osseous involvement and nodal involvement of Level I and II. Since nodal involvement is the single most important prognostic indicator, an accurate assessment of all nodal chains at the same time is essential in staging, treatment, and prognosis (Table 1). Hence, advanced imaging is key in detecting the prognosis of the disease.
| Level of Lymph Nodes | Anatomical Location |
|---|---|
| I | All nodes above the hyoid bone, below the mylohyoid muscle, and anterior to a transverse line drawn on each axial image through the posterior edge of the submandibular gland. |
| II | Skull base, at the lower level of the bony margin of the jugular fossa, to the level of the lower body of the hyoid bone. |
| III | Between the level of the lower body of the hyoid bone and the level of the lower margin of the cricoid cartilage arch. |
| IV | Between the level of the lower margin of the cricoid cartilage arch and the level of the clavicle on each side as seen on each axial scan. |
| V | Skull base, at the posterior border of the attachment of the sternocleidomastoid muscle, to the level of the clavicle as seen on each axial scan. |
| VI | Inferior to the lower body of the hyoid bone, superior to the top of the manubrium, and between the medial margins of the left and right common carotid arteries or the internal carotid arteries. They are the visceral nodes. |
| VII | Caudal to the top of the manubrium in the superior mediastinum, between the medial margins of the left and right common carotid arteries. |
MR imaging findings that are indicative of osseous involvement include loss of low-signal-intensity cortex, replacement of high signal intensity marrow on T1-weighted images by intermediate-signal-intensity tumor, contrast enhancement within the bone, and contrast enhancement of nerves traversing the mandible. In our study, a Head MRI was performed to evaluate the area, but no concurrent findings were noted.
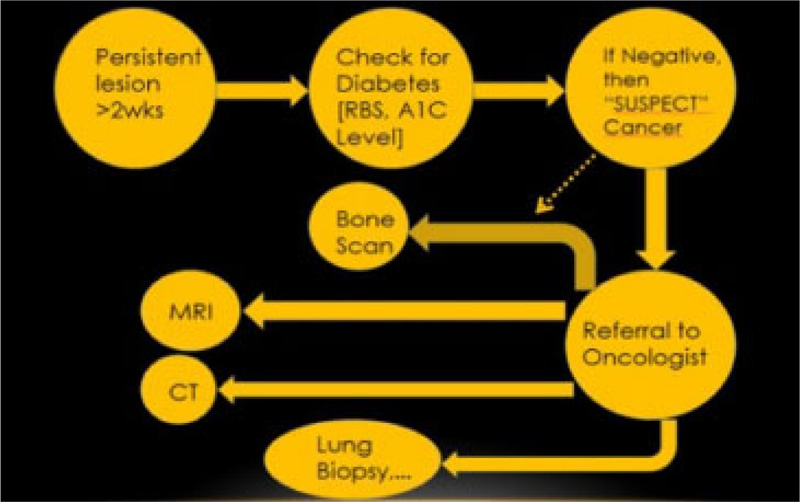
Lubek et al. in 2011, conducted a retrospective analysis of 72 patients with gingival carcinoma and indications for elective neck dissection. They found that elective neck dissection is indicated for all stages of mandibular gingival and T3 or T4 carcinomas of the maxillary gingiva. T2 maxillary SCC should be considered for neck dissection. Overall, disease-free survival was worse among those with cervical metastasis and patients who had marginal resections [11]. In this case, neck dissections were performed but leading up to the current time in the patient’s follow-up, the patient did not respond well to any therapy. Hence, it is noteworthy that conditions like this must be diagnosed as early as possible. Therefore, any persistent lesion exhibiting features that are not responding to conventional gingival and periodontal treatment options for more than two weeks should be referred for further evaluation to rule out cancer (Fig. 9). The 5-year survival rate of gingival SCC is considerably less when compared to SCC developing at other sites, suggesting a poor prognosis [12].
This case highlights the presence of a very serious condition like gingival squamous cell carcinoma (SCC) and its deceptive behavior as a benign and inflammation-mimicking lesion in the gingiva. Many times, clinicians dismiss persistent lesions as idiopathic without adequate investigations, and doing so could result in missing a potentially life-threatening disease like squamous cell carcinoma (SCC).
CONCLUSION
This case report emphasizes that clinicians should pay attention to unresolving lesions, especially those of inflammatory origin, and serves as a reminder that delay in appropriate radiographic examination and diagnosis of these lesions may adversely affect the prognosis.
AUTHORS' CONTRIBUTION
Data acquisition and analysis of data were performed by Dr. Elnaz Jalali. Dr. Jyoti Mago wrote the manuscript and is the corresponding author. Dr. Aditya Tadinada is the guarantor who edited the manuscript and reviewed it.
ETHICS APPROVAL AND CONSENT TO PARTI-CIPATE
This study was approved by the Hanoi National Hospital of Odonto Stomatology, Vietnam (No. 217/HDDD-BVRHMTW).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from patients and their parents for treatment and publication.
STANDARDS OF REPORTING
CARE guidelines and methodologies were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting findings of this study is available from the corresponding author [J.M.], upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


