All published articles of this journal are available on ScienceDirect.
Antibacterial Efficacy of Casein Phosphopeptide-Amorphous Calcium Phosphate Compared to Calcium Hydroxide as Intracanal Medicaments against Enterococcus faecalis: In-vitro Study
Abstract
Background:
Endodontic infection may persist despite root canal instrumentation. Thus, the use of intracanal medicaments plays an essential role in eliminating resistant bacteria like Enterococcus faecalis, known to be associated with persistent infections in endodontically treated teeth. Although calcium hydroxide is the gold standard intracanal medicament, it has been reported that Enterococcus faecalis is immune to its effects. Therefore, several studies assessed the efficacy of other intracanal medicaments, but none to date evaluated Casein Phosphopeptide-Amorphous Calcium Phosphate.
Objectives:
This in-vitro randomized controlled study aimed to assess the antibacterial efficacy of Casein phosphopeptide-amorphous calcium phosphate as an intracanal medicament against Enterococcus faecalis and compared it to calcium hydroxide.
Methods:
60 extracted single root canal permanent teeth were prepared and later divided into three equal groups according to the intracanal medicament used. Group 1: No intracanal medicament (negative control), Group 2: Calcium hydroxide paste, and Group 3: Casein phosphopeptide-amorphous calcium phosphate paste. The intracanal medicaments were placed on the canals for 7 days. The outcome of this procedure was measured by counting colony-forming units. Statistical analysis was carried out using One-Way ANOVA and Tukey’s Post Hoc Test to determine significant differences between the groups.
Results:
The mean bacterial count for Group 2 was significantly lower than Group 1 and Group 3. Calcium hydroxide showed significantly more antibacterial efficacy against Enterococcus faecalis than Casein phosphopeptide-amorphous calcium phosphate and the negative control groups.
Conclusion:
Casein Phosphopeptide-amorphous calcium phosphate is ineffective in inhibiting Enterococcus faecalis growth compared to Calcium hydroxide.
1. INTRODUCTION
Pulpal and periapical diseases develop from bacterial colonization of the root canal system [1, 2]. Non-surgical endodontic therapy targets this pathogenic microflora through instrumentation and obturation [3]. However, due to the anatomical complexities of the root canal system, complete removal of bacteria is not achieved through instrumentation alone. Certain bacterial species such as Enterococcus faecalis (E. faecalis) can survive and thrive in the harsh environment of endodontically treated teeth, perpetuating persistent endodontic infection [4, 5].
This highlights the essential role of intracanal medicaments in eliminating the remaining bacteria and providing a favorable environment for periapical tissue repair [6]. Calcium Hydroxide (CH), due to its bactericidal characteristic, became one of the most widely used intracanal medicaments. It has a destructive effect on their protein structures and cell membranes due to its high pH of around 12.5 [7].
In spite of CH's success, some studies found E. faecalis resistant to its effects [8] and its long-term use associated with root fracture [9]. This has prompted studies to examine other alternatives that can overcome the disadvantages of CH.
During the last decade, Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) proved successful in the prevention and treatment of early carious lesions through its remineralization effect and its moderating influence on acidogenic oral bacteria [10]. CPP-ACP is a nano complex derived from milk protein, formulated from two parts: Casein Phosphopeptide (CPP) and Amorphous Calcium Phosphate (ACP) [10]. Commercially, it is available in the form of a paste - MI Paste Plus (GC America) - that also contains fluoride.
Studies reported that stannous fluoride can inhibit E. faecalis growth [11, 12]. CPP has a bactericidal and buffering effect on plaque, interfering with the growth and adherence of the species Streptococcus mutans and Streptococcus sobrinus [13].
A search conducted through PubMed and Google Scholar search engines, with keywords: CPP-ACP and intracanal medicament, showed no studies evaluating the efficacy of CPP-ACP as an intracanal medicament.
Therefore, this study aimed to assess the antibacterial efficacy of CPP-ACP (MI Paste Plus) against E. faecalis compared to calcium hydroxide, the “gold standard” intracanal medicament. It was hypothesized that CPP-ACP is more effective than CH against E. faecalis.
2. MATERIALS AND METHODS
This in vitro study was conducted at Princess Nourah Bint Abdulrahman University (PNU), Dental College Simulation Lab, and King Saud University, College of Dentistry Research Center, Microbiology Research Lab. Ethical approval was obtained from the Institutional Review Board (IRB) at PNU.
2.1. Sample Selection and Preparation
Sixty single-rooted permanent teeth extracted for periodontal or orthodontic reasons were collected from private and public dental clinics in Riyadh. Exclusion criteria included, previously treated teeth, calcified canals, open apex, root fracture, curved canals, or anatomic malformation. Tissue tags, calculus, or debris present on the external tooth surface were removed to obtain a clean surface. Teeth were stored in normal saline to prevent desiccation.
Each tooth was mounted on an individual block of modeling wax. The crown was removed at the cementoenamel junction using a diamond bur (knife-edge 016, Komet, Germany). A No 15-stainless steel K- file (Medin, A.S. Czechia) was introduced into the canal until it was visible at the apical foramen. This was verified with the aid of dental loupes (JTL Gobiz, Korea x3). Determination of the working length was done by subtracting 0.5 mm from this measurement. Instrumentation was done using ProTaper files (Dentsply Maillefer, OK, USA) according to manufacturer instructions. On a 16:1 contra-angle handpiece attached to an electric motor (X-smart Endodontic Rotary Motor, Dentsply-Sirona, NC, USA). Canals were prepared until size F3 ProTaper file and irrigated with saline in between the instrumentation sequence to remove debris.
2.2. Bacterial Penetration and Inoculation
After instrumentation, teeth were autoclaved twice at 121 degrees Celsius to eliminate all microbes from the teeth, preventing contamination with other microbes. A pure culture of test strain of E. faecalis ATCC 29212 was inoculated in sterile nutrient broth. The presence of E. faecalis was confirmed in the nutrient broth by pipetting 10 microliters of the broth and observing its presence under a microscope. Nutrient broth inoculated with E. faecalis was transferred to sterile containers where the level of the broth was marked. Each extracted tooth was placed in a marked container filled with the nutrient broth containing E. faecalis. The containers were incubated for 14 days at 37 degrees Celsius. The level of the nutrient broth was checked daily, and new broth inoculated with E. faecalis was added to maintain all the extracted teeth completely submerged in the inoculated broth for 14 days.
2.3. Specimens’ Grouping and Intracanal Medicament Application
The inoculated teeth were randomly divided using block randomization lots into three equal groups (n=20), according to the intracanal medicament they will receive. In Group 1, no intracanal medicament was placed in the canals (negative control group). In Group 2, CH injectable paste (Calcicur, VOCO, Cuxhaven, Germany) was placed into the root canals using injectable syringes. In Group 3, CPP-ACP (MI Paste Plus, GC America, Tokyo, Japan) was placed into the root canals using injectable syringes. All canals were dried with paper points prior to placement of the intracanal medicament.
MI Paste Plus has a similar consistency to calcium hydroxide injectable paste. Therefore, to standardize appli- cation, MI Paste Plus was loaded into a syringe with the same design and capacity as the syringe containing the calcium hydroxide paste: A 2.5g Luer lock syringe with a single-use cannula (20 mm long, 0.6 mm diameter), which was changed for each tooth.
2.4. Antimicrobial Assessment
After placement of the intracanal medicament, all teeth were sealed using temporary cement (Cavity-G, 3M ESPE AG, EU), wrapped in moist cotton, and kept in an incubator at 37°C for 7 days. The temporary filling was then removed, and a sample of dentin was taken from each canal to evaluate the colony-forming units. A new sterilized No. 5 Gates Glidden drill (Mani, Inc., Tochigi, Japan) was used to remove the dentin sample from each tooth to prevent cross-contamination. Dentin debris from the Gates Glidden drill was transferred to 1 mL of saline. All the micro-test tubes were kept in a water bath for 30 min at 62°C to prevent cross-contamination. On nutrient agar, 100 microliters of solution were pipetted from each micro test tube and inoculated. This agar was incubated at 37°C, and the colonies of E. faecalis (colony-forming units) were calculated using a colony counter by an experienced microbiologist blinded to the groups. The presence of E. faecalis was verified using eosinophil stain and gentian violet staining to identify gram-positive microorganisms.
2.5. Statistical Analysis
Statistical analysis was performed using the JMP SAS software package (SAS Institute, NC, USA). Bacterial counts were Log transformed to get a more normal distribution, then analyzed using variance test (One-Way ANOVA) and Tukey’s Post Hoc Test to determine significant difference between the groups at a set p-value of ≤ 0.05.
3. RESULTS
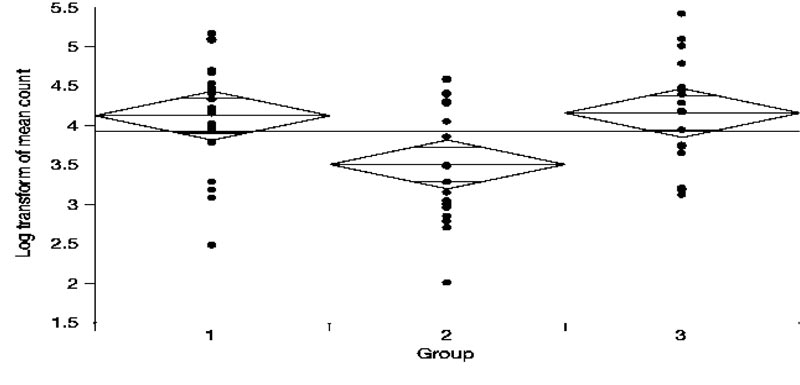
Fig. (1) shows the Log transformed number of colony-forming units seen in each group. The mean bacterial counts for Group 1, Group 2, and Group 3 were 4.12, 3.50, and 4.15, respectively. According to the One-way ANOVA test (Table 1) and Post-hoc comparisons using the Tukey-Kramer HSD test: The mean bacterial count for Group 2 was significantly lower compared to both Group 1 and Group 3. However, Group 1 did not significantly differ from Group 3. (Table 2).
Thus, intracanal medicament using CH was associated with significantly fewer colony-forming units of E. faecalis when compared to CPP- ACP and negative control groups.
4. DISCUSSION
Biocompatible intracanal medicaments complement the disinfection of the root canal system by reducing the number of root canal microorganisms [14]. The results of this study showed that CH intracanal medicament demonstrated maxi- mum microbial inhibition of E. faecalis growth compared to both the CPP-ACP and negative control groups. This is in accordance with a study conducted by Mohammadi et al. that reported the effectiveness of CH in the elimination of E. faecalis from the dentinal tubules when used in aqueous and silicon oil-based pastes [15]. Another study found the application of CH for seven days sufficient in reducing bacterial counts in canals to a negative culture [16].
| Group | Number | Mean | F | Sig. |
|---|---|---|---|---|
| 1 | 20 | 4.1247452 | 5.6296 | 0.0059* |
| 2 | 20 | 3.5068784 | - | - |
| 3 | 20 | 4.1586882 | - | - |
| Group | - Group | Difference | Std Err Dif | Lower CL | Upper CL | p-Value |
|---|---|---|---|---|---|---|
| Group 3 | Group 2 | 0.6518098 | 0.2186965 | 0.125533 | 1.178086 | 0.0116* |
| Group 1 | Group 2 | 0.6178668 | 0.2186965 | 0.091590 | 1.144143 | 0.0176* |
| Group 3 | Group 1 | 0.0339430 | 0.2186965 | -0.492333 | 0.560219 | 0.9868 |
This efficacy is attributed to the release of hydroxyl ions in an aqueous environment that causes damage to the bacterial cytoplasmic membrane, protein denaturation, and bacterial DNA destruction [17].
Many studies were conducted to test the antibacterial efficacy of other materials as intracanal medicaments. For example, Panchal et al. compared cinnamon extract to CH as an intracanal medicament [18]. The present study utilized well-established principles by previous studies: 14 days of bacterial inoculation was carried out to ensure bacterial penetration into the dentinal tubules [19], intracanal medicaments were left in the canals for a period of 7 days to achieve optimal antimicrobial efficacy of CH according to Sjogren et al. [20], colony-forming units were the outcome variable used to discriminate between the antibacterial efficacy of the different materials [18, 19].
Several studies have explored the application of CPP-ACP in endodontics. One study proposed its use as a final root canal irrigant to increase the micro-hardness of root dentin [21], while another examined its use as a root canal sealer [22]. However, no study has explored the use of CPP-ACP as an intracanal medicament by testing its antibacterial effect against E. faecalis.
The CPP-ACP and negative control groups showed no statistically significant difference in E. faecalis growth. Although CPP-ACP was associated with lower bacterial counts of Streptococcus mutans - a facultative anaerobe, Gram-positive bacteria like E. faecalis, this is likely due to its anti-adhesion effects more than any bacterial static or bactericidal effects [23]. Studies conducted on the effect of CPP-ACP on polymicrobial biofilms showed that while certain caries-associated bacterial species were reduced in the count, other health-promoting bacteria such as the Streptococcus sanguinis and Streptococcus mitis, increased in number [24, 25].
Another factor perhaps linked to CPP-ACP’s antibacterial ability is the presence of saliva. The efficacy of CPP-ACP in reducing Streptococcus mutans count or biofilm development was reported either by clinical trials [25-27] or in vitro studies where the specimens were saliva-coated or placed in an artificial saliva medium [28]. A study where specimens were only placed in broth showed that CPP-ACP did not significantly affect bacterial count reduction [29].
Therefore, a better understanding of the factors that influence CPP-ACP’s anti-bacterial properties and how these factors affect its application in endodontic treatment may be necessary.
Although this study showed that CPP-ACP is not effective as an intracanal medicament against E. faecalis, it can be added to the body of evidence that confirms the effectiveness of CH in inhibiting E. faecalis growth.
CONCLUSION
Within the limitation of the present in vitro study, the results show that CPP-ACP has less antibacterial efficacy against E. faecalis when compared to calcium hydroxide.
LIST OF ABBREVIATIONS
| E. faecalis | = Enterococcus faecalis |
| CH | = Calcium Hydroxide |
| CPP-ACP | = Casein Phosphopeptide-Amorphous Calcium Phos- phate |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval was obtained from the Institutional Review Board (IRB) at Princess Nourah Bint Abdul Rahman University, Saudi Arabia (IRB approval number: 18-0303).
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This research was funded by the Deanship of Scientific Research at Princess Nourah Bint Abdulrahman University through the Fast-track Research Funding Program.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


