All published articles of this journal are available on ScienceDirect.
Influence of Removable Denture Cleaning Agents on Adhesion of Oral Pathogenic Microflora In Vitro: A Randomized Controlled Trial
Abstract
Introduction:
Removable dentures are used by 20% of the population. These may be accompanied by denture stomatitis in 15-70% of patients. The choice of the optimal cleansing agent for removable dental prostheses is of high significance.
Aim:
The aim of our research was to study the influence of removable denture cleansing products on the adhesion of microorganisms and yeast.
Materials and Methods:
We manufactured 144 specimens of standardized round shape with a diameter of 10 mm from 4 types of modern polymeric materials used by prosthetic dentistry to produce removable dentures, 12 specimens of each material were placed into suspensions of bacterial cultures of Staphylococcus aureus, Escherichia coli, Candida albicans, then into “ClearaSept” (Test group 1), “Рrotefix active cleanser” (Test group 2), saline solution (Control group), followed by nutrient media. The adhesion index was calculated and analyzed.
Results:
There was no reliable lowering of adhesion index of Staphylococcus Aureus to all materials detected in Test group 1 (U=6, p>0.05 for Bio XS; U=8, p>0.05 for Dental D, Denotokeep Peek, Vertex Rapid Simplified). In Test group 2, the adhesion index of Staphylococcus Aureus reliably decreased to all materials compared to the Control group (U=0, p≤0.01). The adhesion index of Candida albicans and Escherichia coli to all materials in Test group 1 had a minor to moderate reliable reduction compared to the Control group (U=0, p≤0.01). Test group 2 showed a significant reliable decrease in Candida albicans and Escherichia coli adhesion index to all materials in comparison with the Control group (U=0, p≤0.01).
Conclusion:
The research showed an unreliable or minor and moderate reliable decrease in microorganisms adhesion index depending on the microorganism species after treatment of denture material specimens by antibacterial soap “ClearaSept” and a reliable significant decrease in microbial and yeast adhesion after application of Protefix active cleaner solution, which demonstrates a more significant antimicrobial effect in comparison to “ClearaSept” against Staphylococcus aureus, Escherichia coli, and Candida albicans.
1. INTRODUCTION
Currently, the choice of the optimal cleansing agent for removable dental prostheses care, as well as the mode of its application is of high significance. Removable dentures are used by 20% of the population in Russia, as well as in other countries [1, 2]. It may be accompanied by denture stomatitis in 15-70% of patients. This complication arises due to poor oral and prosthesis hygiene, long-term and nocturnal use, smoking, sugar consumption, salivary pH, accumulation of denture plaque, bacterial and yeast contamination of denture surface [3-5].
There is evidence that removable dental prosthetic constructions serve as a reservoir for oral bacteria and yeast [6]. Materials that are used for manufacturing removable prosthesis bases, such as acrylic resins and thermoplastic materials [7-10], interact with the microbiota of the oral cavity and tissues of the prosthetic bed [11-13].
In addition to it, insufficient polishing of the denture surface leads to the adhesion of edible residues, microorganisms and yeast, including Candida albicans, to the surface of a prosthetic construction [14, 15]. It may also reduce the service life of a removable dental prosthesis.
Candida is known to colonize 72% of prosthetic constructions. The overall prevalence of Candida spp. is higher on the surface of removable dental prostheses in patients who suffer from denture stomatitis (78%), while it is only 64% in their healthy counterparts. Candida albicans is predominant on removable prosthetic constructions in case of denture stomatitis present among other species [16]. Microorganisms and yeast inhabiting dentures may cause not only local but also systemic infections [17].
Consequently, there are several essential aspects of minimizing the risk of denture stomatitis and further possibility of sequelae, including correct choice of denture material and its polishing technique. Type and mode of use of cleansing and disinfecting products for care of removable dentures are essential [18, 19].
Nowadays, mechanical cleaning by toothpaste and brush is common among dental prosthesis users. Treatment of a denture by tooth-powder leads to an increase in surface roughness, retention of edible residues and microorganisms due to its high abrasiveness. Practical dentists may alternatively recommend washing removable prostheses with a soap solution after each food intake. Additionally, cleansing of a denture should be fulfilled regularly by placing the construction in a special solution (Рrotefix, President, ROCS, Сorega) for 15 minutes [20]. Y. Hayran and co-authors (2018) recommend increasing the concentration of the cleansing solution and lengthen the exposure time to 60 minutes in order to secure a good cleaning effect [21]. On one hand, concentration and exposure time modify the antimicrobial effect. On the other hand, usage of cleansing solutions for several hours may damage denture basis in a long term period [22].
Therefore, research of various antibacterial products for denture hygiene care and their impact on growth and reproduction of microorganisms and yeast, as well as their effect on prosthesis chemical structure, is a significant contemporary issue.
Thus, the aim of our research was to study the influence of different products of hygienic care to removable dental prostheses, which are manufactured from various thermoplastic materials and acrylic plastics, in terms of microorganism and yeast adhesion.
2. MATERIALS AND METHODS
Several investigations were performed previously concerning the antimicrobial effect of cleaning agents for dentures, including 0.25% sodium hypochlorite, 0.5% sodium hypochlorite, 1% sodium hypochlorite, 2% chlorhexidine, 2% glutaraldehyde, 3.8% sodium perborate, 0.5% chloramine-T, 0.2% peracetic acid, vinegar-hydrogen peroxide, vinegar-water and hydrogen peroxide-water mixtures at several concentration ratios, 2% aalam extract, and 2% neem extract, 10% Ricinus communis oil, NitrAdine, Efferdent Plus and Corega Tabs, propolis solution for cleaning complete dentures, alkaline peroxide [17, 22-27]. Further investigation of the antimicrobial effect of Protefix in comparison with an antibacterial liquid soap is necessary.
2.1. Trial Design
The current research was conducted in the Testing Laboratory Center of Federal Budget Institution of Health “Center of Hygiene and Epidemiology in the City of Moscow”. The trial was planned as a randomized parallel-group controlled investigation, with the aimed allocation ratio 1:1:1. It was performed in the year 2018, following the Consolidated Standards of Reporting Trials (CONSORT) guidelines [28].
The null hypothesis was that antibacterial liquid soap “ClearaSept” and solution of an active cleaner for dentures «Рrotefix» have an equal antibacterial effect on acrylic removable dentures.
Antibacterial liquid soap “ClearaSept” ® (SwissPharm SA, Geneva, Switzerland) (Test group 1) and Protefix® Active Cleanser (Helago-Pharma GmbH, Erftstadt, Germany) (Test group 2) were chosen for this research. Criteria of choice included their popularity, according to scientific literature and among practical doctors and patients in individual removable denture care, safety, no toxicity, minimal chemical effect on the denture material, and availability to the patient. Saline solution was used as a control (Control group).
Protefix® Active Cleanser is composed of Potassium Caroate, Sodium Bicarbonate, Sodium Carbonate, Citric Acid, Sorbitol, VP/VA Copolymer, Sodium Lauryl Sulfate, Sodium Lauryl Sulfoacetate, Aroma, and CI 73015.
“ClearaSept” consists of Aqua, Sodium Laureth Sulfate, Sodium Chloride, Undecylenamidopropyltrimonium Methosulfate, Glycerin, Cocamidopropyl Betaine, Sodium Hydroxymethylglycinate, Cocamide Dea, Peg-7 Glyceryl Cocoate, Propylene Glycol, Ethylhexylglycerin, Octenidine Hcl, Citric Acid, Peg-120 Methyl Glucose Dioleate, Parfum, and Leptospermum Scoparium (manuka) Oil.
We manufactured 144 specimens of standardized round shape with a diameter of 10 mm and thickness of 1 mm from modern polymeric materials, which are used by prosthetic dentistry to produce removable dentures: Acetal Resin DENTAL D® (QuattroTi S.r.L. Divisuine Tecnopolimeri Biomedicali, Rovello Porro (CO), Italy) (36), Bio XS (Bredent, Moscow, Russia) (36), Dentokeep Peek (NT-trading GmbH & Co., Karlsruhe Germany) (36), and Vertex rapid simplified (© Vertex-Dental B.V. Headquarters The Netherlands) (36).
Polishing of specimens surface was performed according to recommendations of manufacturing companies until the surface reached the state of gloss, which was determined visually and estimated by means of scanning electron microscopy by electron microprobe JXA-8100 (JEOL Ltd., Tokyo, Japan) at 100x, 200x, and 400x magnification.
2.2. Microbiological Analysis
Then 12 specimens of each material were placed into suspensions of bacterial cultures Staphylococcus aureus (strain No. 6538-Р АТСС ®, Manassas, Virginia, U.S.), Escherichia coli (strain No. 25922 АТСС®, Manassas, Virginia, U.S.), Candida albicans (strain No. 25922 АТСС®, Manassas, Virginia, U.S.) in vials.
Criteria for including microorganisms in the study were as follows: presence in the oral cavity of patients using removable dentures, inhabiting dental prostheses. The same species were used for testing the antimicrobial properties of dental prostheses in vitro, previously by other researchers [29, 30].
The number of microorganisms in 1 ml of suspension was 108 CFUs/ml. Exposure time was 1 hour. Further, all specimens were rinsed by sterile water for a duration of 3 minutes. Then 4 specimens of each material were inserted from each suspension of microorganisms into glass tubes containing specific liquid for care for removable dentures. Thus, 48 specimens underwent submersion in “ClearaSept” (Test group 1) and 48 specimens were placed into a solution of “Рrotefix active cleanser” (Test group 2) with exposure for 15 minutes. The control group consisted of 48 specimens that were put into a saline solution.
After that, the specimens of materials were placed into nutrient media, including 16 specimens from each solution into Sabouraud Dextrose Agar medium (monitoring of the growth of Candida albicans) and 32 from each solution into 2% liquid nutrient medium (monitoring of the growth of Escherichia coli and Staphylococcus aureus), with exposure in a thermostat UT-2035 (ULAB™, China) at temperature 37 °C during 24 hours. Then seeding germs from each nutrient medium to each dense nutrient media was accomplished onto Endo agar for Escherichia coli, vitelline-saline agar for Staphylococcus aureus, and Sabouraud agar for Candida albicans.
While studying the adhesion of microorganisms, we based our research on the methodology of V.N. Tsarev (2006). It allows comparing the number of bacteria in a test culture, which was put onto a specimen of a material, with the amount of adhered bacteria per 1 cm2 [31]. After cultivation, the number of isolated colonies was counted, which grew from bacteria having adhered to a specimen of a material; thereafter this number was calculated per 1 cm2 of the specimen. Obtained results were expressed through a decimal logarithm (lg) of a number of Colony-forming Units (CFUs).
The adhesion index was calculated according to a formula. This index was expressed as a quotient from dividing the obtained value by the decimal logarithm of the concentration of bacteria (yeast) in initial suspension, which was placed onto a specimen of examined material. Ia= lg A/ lg N, where Iа is adhesion index; A is the number of adhered bacteria counted during microscopy; N is the initial quantity of bacteria in suspension.
2.3. Outcomes
The outcome we aimed to get was the value of adhesion index of Staphylococcus aureus, Escherichia coli, and Candida albicans to various polymers after treatment with antibacterial soap liquid, Protefix, and saline solution.
2.4. Sample Size
The study was designed to detect a difference in the number of bacteria of at least 17200.00 CFU between samples that underwent treatment using different types of cleansing agents (SD 90000.00±30000.00 CFU, retrieved from the investigation performed by L. Gupta and co-authors (2017) [32]. The number of specimens for each group was calculated to be 48 (alpha = 0.05; power = 80%). A total of 144 specimens were included (48 in each group).
2.5. Randomization and Allocation
The solutions were dispensed in and delivered without specific identification. Each cleanser solution was used by the participants in a random sequence that was determined using a computer. Researcher P1 manufactured and polished the specimens, followed by obtaining the list of random numbers for specimens by Researcher P2 using a computer program and gathering the data to perform statistical analysis. Then Researcher P3 investigated and registered microbiological data, while Researcher P4 placed the specimen into bacterial cultures. Researchers P5 and P6 inserted the specimens into antibacterial solutions in identical dark flasks and placed the samples into nutrient media correspondingly. All the data was gathered by the blinded researcher at each stage and transmitted to P2, who performed the statistical analysis.
2.6. Blinding
Researcher P1 was blinded to the numbers given to the samples. Statistical analysis was performed by Researcher P2, who was masked to the type of the sample and to the name of liquid and of bacterial culture. Researchers P3, P4, P5, and P6 were blinded to the denture material and to previous and following steps of investigation for each specimen, and Researcher P2 could not have an impact on the procedures and data gathering.
2.7. Statistical Methods
Statistical analysis of results was performed by means of generally accepted statistical methods using the standard block of statistical programs Microsoft Excel ® (2007) (Microsoft Corporation, Redmond, WA, U.S.) and SPSS Statistics 23 (SPSS Inc., Chicago, IL, U.S.). We calculated the mean value (M), standard deviation (m), median, minimum, maximum, level of confidence in order to describe the quantitative attributes. Mann-Whitney parameter was used for pairwise comparison of independent samples.
3. RESULTS
The trial was accomplished according to the initial design and was finished after the acquirement of the necessary data. Test groups 1 and 2 and the control group included 48 specimens in 1 group that was divided into 3 subgroups according to microorganism or yeast microbiological study, containing 4 specimens that were manufactured of each investigated material.
The results of assessing microorganism adhesion values to the surface of polymer specimens for Test groups and Control group are presented in Tables 1, 2, 3, and 4 and are illustrated in Fig. (1).
| Test Group 1 (n=48) | Test Group 2 (n=48) | Control Group (n=48) | |||||
|---|---|---|---|---|---|---|---|
| Adhesion to Microorganism/yeast | S. aureus (n=16) | C. albicans (n=16) | E. coli (n=16) | S. aureus (n=16) | C. albicans (n=16) | E. coli (n=16) | |
| Mean Value of Ia (M) | 0,42 | 0.36 | 0.41 | 0,16 | 0.17 | 0.15 | 0,42 |
| Standard Deviation (m) | 0.005 | 0.007 | 0.006 | 0,023 | 0.04 | 0.027 | 0 |
| Median | 0,42 | 0,36 | 0,41 | 0,16 | 0,16 | 0,14 | 0,42 |
| Minimum | 0,41 | 0,35 | 0,40 | 0,12 | 0,11 | 0,12 | 0,42 |
| Maximum | 0,43 | 0,37 | 0,42 | 0,19 | 0,29 | 0,19 | 0,42 |
| Confidence level 95% | 0,0028 | 0,004 | 0,0036 | 0,0143 | 0,00249 | 0,00168 | - |
| Material | Test Group 1 (n=16), Adhesion Index | Test Group 2 (n=16), Adhesion Index | Control group (n=16), Adhesion index |
Mann-Whitney Parameter, U and Significance, P Test group 1/ Control group Test group 2/Control Group Test group 1/Test group 2 |
|---|---|---|---|---|
| Bio XS (n=12) | 0.42±0.003 | 0.12±0.003 | 0.42±0.0 | U=6, P>0.05 U=0, P≤0.01 U=0, P≤0.01 |
| Dental D (n=12) | 0.42±0.003 | 0.16±0.003 | 0.42±0.0 | U=8, P>0.05 U=0, P≤0.01 U=0, P≤0.01 |
| Dentokeep Peek (n=12) | 0.42±0.005 | 0.16±0.003 | 0.42±0.0 | U=8, P>0.05 U=0, P≤0.01 U=0, P≤0.01 |
| Vertex Rapid Simplified (n=12) | 0.42±0.006 | 0.18±0.003 | 0.42±0.0 | U=8, P>0.05 U=0, P≤0.01 U=0, P≤0.01 |
| Total (n=48) | 0.42±0.005 | 0.16±0.023 | 0.42±0.0 | U=120, P>0.05 U=0, P≤0.01 U=0, P≤0.01 |
| Material | Test Group 1 (n=16), Adhesion Index | Test Group 2 (n=16), Adhesion Index | Control Group (n=16), Adhesion Index |
Mann-Whitney Parameter, U and Significance, P Test group 1/ Control group Test group 2/Control Group Test group 1/Test group 2 |
|---|---|---|---|---|
| Bio XS (n=12) | 0.36±0.008 | 0.16±0.073 | 0.42±0.0 | U=0, P≤0.01 U=0, P≤0.01 U=0, P≤0.01 |
| Dental D (n=12) | 0.36±0.003 | 0.18±0.007 | 0.42±0.0 | U=0, P≤0.01 U=0, P≤0.01 U=0, P≤0.01 |
| Dentokeep Peek (n=12) | 0.36±0.007 | 0.16±0.006 | 0.42±0.0 | U=0, P≤0.01 U=0, P≤0.01 U=0, P≤0.01 |
| Vertex Rapid Simplified (n=12) | 0.36±0.006 | 0.19±0.003 | 0.42±0.0 | U=0, P≤0.01 U=0, P≤0.01 U=0, P≤0.01 |
| Total (n=48) | 0.36±0.007 | 0.17±0.04 | 0.42±0.0 | U=0, P≤0.01 U=0, P≤0.01 U=0, P≤0.01 |
| Material | Test Group 1 (n=16), Adhesion Index | Test Group 2 (n=16), Adhesion Index | Control Group (n=16), Adhesion Index |
Mann-Whitney Parameter, U and Significance, P Test group 1/ Control group Test group 2/Control Group Test group 1/Test group 2 |
|---|---|---|---|---|
| Bio XS (n=12) | 0.41±0.006 | 0.12±0.003 | 0.42±0.0 | U=0, P≤0.01 U=0, P≤0.01 U=0, P≤0.01 |
| Dental D (n=12) | 0.41±0.005 | 0.18±0.003 | 0.42±0.0 | U=0, P≤0.01 U=0, P≤0.01 U=0, P≤0.01 |
| Dentokeep Peek (n=12) | 0.41±0.006 | 0.12±0.003 | 0.42±0.0 | U=0, P≤0.01 U=0, P≤0.01 U=0, P≤0.01 |
| Vertex Rapid Simplified (n=12) | 0.41±0.005 | 0.16±0.005 | 0.42±0.0 | U=0, P≤0.01 U=0, P≤0.01 U=0, P≤0.01 |
| Total (n=48) | 0.41±0.006 | 0.15±0.027 | 0.42±0.0 | U=0, P≤0.01 U=0, P≤0.01 U=0, P≤0.01 |
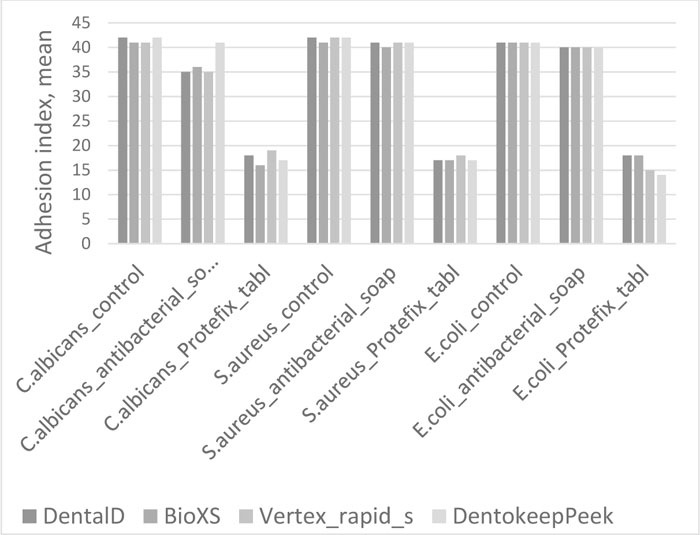
There was no reliable lowering of adhesion index of Staphylococcus Aureus to all materials detected in Test group 1 (U=6, p>0.05 for Bio XS; U=8, p>0.05 for Dental D, Denotokeep Peek, Vertex Rapid Simplified).
In Test group 2, the adhesion index of Staphylococcus Aureus reliably decreased to all materials compared to the Control group (U=0, p≤0.01 for Bio XS, Dental D, Denotokeep Peek, Vertex Rapid Simplified). The minimal value of adhesion index was observed by material Bio XS (0.12±0.003).
The adhesion index of Candida albicans to all materials in Test group 1 has moderate reliable reduction compared to the Control group (U=0, p≤0.01 for Bio XS, Dental D, Denotokeep Peek, Vertex Rapid Simplified). Test group 2 showed a significant reliable decrease in adhesion index in comparison with the Control group (U=0, p≤0.01 for Bio XS, Dental D, Denotokeep Peek, Vertex Rapid Simplified).
Test group 1 demonstrated a reliable minor adhesion index reduction of Escherichia coli to all materials (U=0, p≤0.01 for Bio XS, Dental D, Denotokeep Peek, Vertex Rapid Simplified). A significant reliable decrease in Escherichia coli adhesion was observed in Test group 2 to all materials, most prominently in Bio XS and Dentokeep Peek (U=0, p≤0.01 for Bio XS, Dental D, Denotokeep Peek, Vertex Rapid Simplified).
4. DISCUSSION
4.1. Limitations
The study has several limitations.
The research does not study all possible species of microorganisms that may inhabit removable dentures.
The current trial does not take into account the possible own antibacterial properties of removable denture materials.
No polishing technique influence was discussed in this paper.
In addition, the investigation did not aim to compare other agents that may be used as cleansers for removable dental prostheses apart from the agents that underwent current research.
4.2. Generalisability
Microbiota of the oral cavity is specific in patients with removable dentures. The denture-associated oral microbiota was studied in healthy patients and people suffering from stomatitis by B. Shi and co-authors (2016), who found the most common bacteria to be Actinomyces, followed by Streptococcus, Veillonella, Capnocytophaga, Neisseria, Prevotella, Corynebacterium, Rothia, Leptotrichia, Porphyromonas, Selenomonas, Campylobacter, Lautropia, Granulicatella, Haemophilus, Kingella, Fusobacterium, Bacteroidales, Actinobaculum, Tannerella, Clostridiales, Gemella, Eikenella and Abiotrophia [33].
However, some microorganisms and yeast should be specially marked. Candida albicans is not only able to adhere to the mucous surfaces but also fixate to the acrylic resins of the dental prostheses. Both the plaque accumulated on the denture and the poor oral hygiene contribute to the virulence of Candida, offering the clinical picture of Candida-associated denture stomatitis [34]. On the other hand, Staphylococcus aureus is not only known to form poor monoculture biofilms in serum but is also able to produce a substantial polymicrobial biofilm in the presence of Candida albicans. In terms of architecture, Staphylococcus aureus formed microcolonies on the surface of the biofilm, with Candida albicans serving as the underlying scaffolding. In addition, Staphylococcus aureus matrix staining revealed a different phenotype in polymicrobial versus monomicrobial biofilms, suggesting that Staphylococcus aureus may become coated in the matrix secreted by Candida albicans [35].
Escherichia coli is generally not as frequently found in oral microbiota but is more often present in patients with removable dentures, which is due to significant dysbiosis and leads to the necessity of removing these bacteria from the denture. Moreover, Staphylococcus aureus and Escherichia coli are typical Gram-positive and Gram-negative bacterial strains [30].
The in vitro antimicrobial property of removable dentures was earlier determined by a test against Escherichia coli, Staphylococcus aureus and Candida albicans by Mirizadeh A. and co-authors (2018) and Jabłońska-Stencel E. and co-authors (2018) [29, 30].
Both antibacterial properties of removable prostheses and their cleansers are important contemporary issues of prosthetic dentistry. Sodium hypochlorite is routinely recommended for cleaning dentures. Although it is proven to be effective, its use is limited because it whitens acrylic resins and corrodes metal components of prostheses [17, 36, 37]. Despite the fact that rinsing and antimicrobial solutions are vital for cleaning and bacterial control of dentures, their application for several hours daily may damage the dentures [21, 22]. Other rinsing liquids were investigated for antibacterial and chemical properties. Oil derived from a castor bean (Ricinus communis) is biocompatible and has bactericidal and fungicidal effects [38]. Antibacterial properties of aalam extract and neem extract in denture cleansing were also investigated [25].
Several investigations were performed previously by different authors concerning the effect of cleaning agents on individual denture hygiene. M. Salles and co-authors (2015) compared the antimicrobial effect of 0.25% sodium hypochlorite, 0.5% sodium hypochlorite, 10% Ricinus communis oil, and 0.85% saline against specific microorganisms, including Streptococcus mutans, Candida spp., and gram-negative microorganisms and found that the 0.5% sodium hypochlorite solution was the most effective cleanser and might be used to control denture biofilm [17]. R.F. de Souza and co-authors (2019) presented their investigation of a propolis solution efficacy for cleaning complete dentures, comparing to saline or alkaline peroxide, to Staphylococcus aureus, Streptococcus mutans, Escherichia coli, Candida albicans, Candida glabrata, Candida parapsilosis [24]. Andonissamy L. and co-authors (2019) compared the disinfecting effect of 1% sodium hypochlorite, 2% chlorhexidine, 2% glutaraldehyde, 3.8% sodium perborate, 2% aalam extract, and 2% neem extract against Staphylococcus aureus and viridans Str. Spp. from acrylic dentures, which resulted in the fact that the use of 2% glutaraldehyde was the most effective measure [25]. The effects of three disinfection protocols on Candida spp., denture stomatitis, and biofilm were investigated by M.M. Badaró and co-authors (2020), who stated that 0.25% sodium hypochlorite was more effective in comparison with 10% Ricinus communis and 0.5% chloramine-T [26]. O.Ozyilmaz, C. Akin (2019) investigated the effect of four dental cleaners, including Corega and Protefix, on denture base resins' structural properties and concluded that denture cleansers can considerably alter the surface roughness and hardness of denture base resins and should be used carefully depending on the material, but their study did not reflect the antimicrobial aspect [39].
4.3. Interpretation
Our study aimed to compare the antimicrobial action of Protefix and antibacterial liquid soap “ClearaSept”, studying it through the effect to Staphylococcus aureus, Escherichia coli, and Candida albicans. The null hypothesis that antibacterial liquid soap “ClearaSept” and solution of an active cleaner for dentures Рrotefix have an equal antibacterial effect on removable acrylic dentures was rejected.
Results showed that no reliable reduction of adhesion index of Staphylococcus aureus was detected after treatment of specimens with antibacterial liquid soap “ClearaSept”, while there was a minor reliable decrease in Candida albicans adhesion index and moderate reliable reduction of it for Escherichia Coli. The acquired data allows to state that removable denture care with antibacterial liquid soap “ClearaSept” does not have significant antibacterial action on the dental prosthesis.
Protefix Active Cleanser demonstrated significantly different results for all microorganisms and yeast. The adhesion index of Staphylococcus aureus, Escherichia coli and Candida albicans to all materials reliably decreased after treatment of specimens by a solution of Protefix Active Cleanser. Remarkably, the minimal value of Staphylococcus aureus adhesion index was observed to material Bio XS, which also demonstrated small values of adhesion index for Candida albicans and Escherichia coli. Adhesion index of all microorganisms and yeast also showed smaller values for material Dentokeep Peek, whereas adhesion of bacteria and yeast to Vertex rapid simplified was comparatively higher after treatment by Protefix than to other materials, but significantly lower than after application of the tested antibacterial soap (Figs. 2 and 3).
The results of the current investigation concerning the effect of Protefix on Candida albicans are supported by previous research. Antifungal properties of enriching dental adhesive Protefix were studied by S. Matalon and co-authors (2017), who demonstrated that it decreased Candida albicans growth compared to the control, which is most prominent in the first 24 hours [40].
Differences in anticandidal activity for various denture materials also correspond to the data acquired by Y. Hayran and co-authors (2018), who investigated the effect of denture cleansers on Candida albicans. According to their study, Polident 3 min™ and Corega™ tablets significantly inhibited (p<0.05) the proliferation of Candida albicans against all denture resins at 27-37 mg/mL. Scanning electron microscopy results indicated that there was no significant difference among resin specimens regarding biofilm formation on dentures. The authors state that the polarity value of the resins was statistically associated with their anticandidal activity [21], and this data is supported by our results.
Comparative evaluation of results shows that after treatment by antibacterial soap “ClearaSept” and solution of active cleaner Protefix, the adhesion indexes of selected test cultures differ. A minor decrease in adhesion indexes after treatment of specimens by the investigated antibacterial soap may be evidence of its insufficient effectiveness in cleansing removable dental prostheses. On the contrary, a reliable decrease in adhesion indexes for the solution of active cleaner Protefix proves the advantages of treating removable dentures by it. It allows recommending Protefix as a better cleansing and disinfecting solution for the care of removable dental prostheses than the antibacterial soap “ClearaSept”.

CONCLUSION
The research showed an unreliable or minor and moderate reliable decrease in microorganisms adhesion index depending on the microorganism species after treatment of denture material specimens by antibacterial soap “ClearaSept” and a reliable significant decrease in microbial and yeast adhesion after application of Protefix active cleaner solution, which demonstrates a more significant antimicrobial effect in comparison to “ClearaSept” against Staphylococcus aureus, Escherichia coli and Candida albicans.
LIST OF ABBREVIATIONS
| CFUs | = Colony-forming Units |
| Ia | = Adhesion index |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The authors confirm the trial was only conducted in vitro, the Clinical Trial Registration Number is No 09-18, from 10.10.2018, given by Local Ethical Committee of Sechenov University, Russian Federation.
HUMAN AND ANIMAL RIGHTS
No animals/ humans were used for studies that are basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [S.I.R], upon reasonable request.
FUNDING
Supported by the “Russian Academic Excellence Project 5-100”.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


