All published articles of this journal are available on ScienceDirect.
Is An Orthodontic Hydrophilic Composite Resistant to Contamination and pH Cycling? In vitro Results
Abstract
Objective:
To evaluate the adhesive strength of a hydrophilic composite submitted to contamination and pH cycling, compared to a conventional composite.
Materials and Methods:
Seventy-two bovine incisors were prepared and randomly divided into 6 groups (n=12), bonded with Hydrophilic Composite (HC)(Transbond Plus Color Change) or with Conventional Composite (CC)(Transbond XT; control), with or without contamination and pH cycling as follows: G1-HC, with contamination, with pH cycling; G2-HC, with contamination, without pH cycling; G3-HC, without contamination, with pH cycling; G4-HC, without contamination, without pH cycling; G5-CC, without contamination, with pH cycling; G6-CC, without contamination, without pH cycling. Contamination in G1 and G2 consisted of fresh saliva applied after the self-etching primer for 5min before bonding with HC. After bonding, G1, G3, and G5 were submitted to pH cycling, immersed in the demineralizing solution for 22h and for 2h in remineralizing artificial saliva, repeated for 15 days. G2, G4, and G6 were kept in deionized water. The shear bond strength was tested using a load cell of 100N and the Adhesive Remnant Index (ARI) was assessed. Intergroup comparison was performed with one-way ANOVA, Tukey, and chi-square tests.
Results:
There was a statistically significant difference in G1, G2, and G3 in relation to G6. The highest rate of adhesive failure between the resin/bracket interface occurred with the HC, while CC failed more at the resin/tooth interface.
Conclusion:
Contamination and pH cycling did not decrease the shear bond strength of brackets bonded with the hydrophilic composite. However, the adhesive strength of the conventional composite was higher.
1. INTRODUCTION AND STATEMENT OF THE PROBLEM
During orthodontic treatment, bonding of accessories to teeth enamel is usually necessary, interfering in the proper hygiene, retaining biofilm, and increasing the risk of developing dental caries [1]. The studies show a great variation in the prevalence of white spot lesions during orthodontic treatment [2-4], reaching 97% of cases showing one or more lesions at the end of orthodontic treatment [3]. White spot lesions appear in the first six months after the start of fixed orthodontic treatment [2, 5], indicating how quickly decalcification occurs and can become irreversible, progressing to tooth decay.
Bonding with conventional composites requires a sensitive and multi-step technique, which must be orderly and judiciously followed, aiming not to compromise the adhesive resistance of the orthodontic accessories to the tooth enamel, requiring an operative field, free of moisture and without contamination [6].
Currently, hydrophilic composites are used to produce proper adhesion, even in the presence of moisture and contamination [7, 8]. These adhesives have acetone or ethanol as solvents that can move and diffuse through the biofilm to reach hydroxyapatite and promote adequate adhesion after polymerization. Studies have shown that the adhesion of the hydrophilic resin Transbond Plus Color Change, in the presence of contamination, is superior to that of the conventional Transbond XT resin [7], while others have not found this difference [9].
The hydrophilic composite Transbond Plus Color Change presents in its composition glass fluorosilicate as a fluoride source and the hydrophilic nature of the adhesive allows the diffusion of fluoride through the reticulated matrix cured in an aqueous medium [10]. The preventive potential for white spots of this material was little studied so far and has shown satisfactory results in vitro and in vivo [11, 12].
Romano et al. [13] evaluated in vivo the failure rate of metal brackets bonded with Transbond XT and Transbond Plus Color Change. Failure rates were recorded over six months. At the end of the evaluation, there were only 3 bonding failures in each group, indicating similar adhesive strength of both composites used.
Lon et al. [14] evaluated the shear strength of orthodontic ceramic brackets bonded with Transbond XT and Transbond Plus Color Change in bovine enamel, contaminated or not by saliva, in addition to analyzing the location of the adhesive failure. Saliva contamination decreased the shear bond strength of ceramic brackets bonded with the conventional Transbond XT hydrophobic resin. On the other hand, the use of the hydrophilic resin Transbond Plus Color Change associated with the Self Etching Primer, in an environment contaminated by saliva, provided adequate adhesive resistance for clinical use [14].
Despite these studies, there is no scientific evidence on contamination associated with the cariogenic challenge (pH cycling), controlled and without the influence of other biases.
This way, this study aimed to compare the shear bond strength and the Adhesive Remnant Index (ARI) of orthodontic metallic brackets bonded with a conventional and a hydrophilic composite after contamination and pH cycling.
2. MATERIALS AND METHODS
This study was approved by the Human Research Ethics Committee of Ingá University Center Uningá, Maringá, Brazil (n. 3394761).
The sample consisted of bovine incisors, cleaned and disinfected with 0.1% thymol [7]. The teeth were evaluated under a 10x magnifying glass and those with cracks or enamel defects were excluded.
A sample size calculation was performed using the formula for comparing means, adopting a confidence level of 80% and a maximum acceptable error of 5%, taking into account data from previous research [15], resulting in 12 specimens per group.
In a precision cutter (Isomet 1000, Buehler, Lake Bluff, USA), the tooth crown was sectioned, obtaining 6x6 mm enamel/dentin blocks, and included in PVC tubes (2cm in diameter; 2.5cm high) with acrylic resin chemically activated, so that the dental enamel was flat on the surface.
After this procedure, the included blocks were submitted for finishing and polishing procedures, using decreasing-grained silicon carbide sandpaper (#600 211Q, and #1200, 401Q, 3M, São Paulo, Brazil) in a metallographic polishing machine (Aropol-2V, São Paulo, Brazil). Between each stage of polishing, the samples were submitted to an ultrasonic vat for cleaning.
After preparation, the specimens were randomly divided into 6 experimental groups (n=12), bonded with a hydrophilic composite (Transbond Plus Color Change, 3M/Unitek, Monrovia, USA) or with a conventional composite (Transbond XT, 3M/Unitek, Monrovia, USA) as control, with or without contamination and pH cycling as follows:
- G1- bonding with hydrophilic composite, with contamination, with pH cycling
- G2- bonding with hydrophilic composite, with contamination, without pH cycling
- G3- bonding with hydrophilic composite, without contamination, with pH cycling
- G4- bonding with hydrophilic composite, without contamination, without pH cycling
- G5- bonding with conventional composite, without contamination, with pH cycling
- G6- bonding with conventional composite, without contamination, without pH cycling.
Enamel prophylaxis was performed with a rubber cup, pumice stone, and water for 10 seconds. Each rubber cup was used on only 4 specimens, the surface was washed with air-jet and distilled water for 10 seconds, and dried with a paper tissue.
After prophylaxis, brackets were bonded, respecting the specifications of each experimental group (Fig. 1). Metal brackets for mandibular incisors were used (Roth, Morelli, São José do Rio Preto, Brazil).


For bonding of brackets in G1 and G2, the self-etching primer (SEP, 3M/Unitek, Monrovia, USA) was applied to the dental surface with a micro brush, by friction, for 5 seconds. Right after, freshly collected saliva, obtained from a single donor, was applied with a micro brush for 5 minutes, and then the brackets were bonded with the hydrophilic composite, Transbond Plus Color Change (3M/Unitek, Monrovia, USA). The excess was removed with an explorer probe, light-cured with halogen light (Poly Wireless, Kavo, Joinville, Brazil), with an irradiance of 1100mW/cm2 for 10 seconds on each side, at a distance of 3mm between the light beam and the bracket, according to manufacturer's instructions.
The brackets of G3 and G4 were bonded with the same hydrophilic composite following the same steps described above, except for the saliva contamination.
In G5 and G6, the enamel was conditioned with 37% phosphoric acid for 30 seconds, washed for 30 seconds, and dried for 10 seconds. A primer adhesive (Transbond XT, 3M/Unitek, Monrovia, USA) was applied to the dried enamel surface, and brackets were bonded with the conventional composite (Transbond XT, 3M/Unitek, Monrovia, USA). The excess of resin was removed with an explorer probe and the resin was light-cured with the same device and protocol described above, following the manufacturer's instructions.
G1, G3, and G5 were subjected to pH cycling (Fig. 2) [16]. The specimens were immersed for 22 hours in demineralizing solution (3mmol/L of calcium, 3mmol of phosphate, 50mL/L of acetic acid, and 0.308g of ammonium acetate, adjusted to pH 4.5 or 5.5 with sodium hydroxide) [11, 17, 18]. After that, they were washed with deionized water, kept for 2 hours in contact with remineralizing artificial saliva (1.54mmol/L of calcium, 1.54mmol of phosphate, 20mmol/L of acetic acid, and 0.308g of ammonium acetate, adjusted to pH 7.0 with potassium hydroxide), completing a 24h cycle. During the pH cycling period, the specimens were kept in an incubator at a constant temperature of 37°C to simulate the oral environment. This dynamic process was repeated for 15 days, with artificial saliva being replaced every two days. G2, G4, and G6 were not submitted to a demineralizing solution but kept only in deionized water, which was replaced every two days [19].
After these 15 days, specimens were sheared in a Universal Testing Machine (EMIC DL500, São José dos Pinhais, Brazil), at a constant speed of 1mm/min [20] (Fig. 3). A 500N load cell was connected to the computer, which recorded the shear bond strength in MPa of each specimen, considering the base area of each bracket [21, 22].
After the shear bond strength, the enamel surface and the brackets were examined using a stereoscopic magnifying glass to verify the Adhesive Remnant Index (ARI) [23]. The remaining adhesive was scored from 0 to 3; score 0 indicates no resin adhered to the enamel surface; score 1 indicates that less than half of the resin had adhered to the enamel surface; score 2 indicates that more than half of the resin adhered to the enamel; and 3 indicates that all the resin adhered to the tooth, including the impression of the bracket mesh [14, 23].
2.1. Statistical Analysis
The shear strength values were subjected to Shapiro-Wilk tests, main effects ANOVA (considering three independent variables: composite, contamination, and pH cycling), one-way ANOVA, and Tukey tests. To check the differences of the ARI among the groups, the chi-square test was used. The data were analyzed using the statistical program SPSS-IBM 22.0 (version 22.0, IBM, Armonk, NY, USA), with a significance level of 5%.

3. RESULTS
The results of the main effects ANOVA showed that the composite was the only variable responsible for differences in the shear bond strength values (p=0.008), while variables contamination (p=0.089) and pH cycling (p=0.052) were not significant (Table 1).
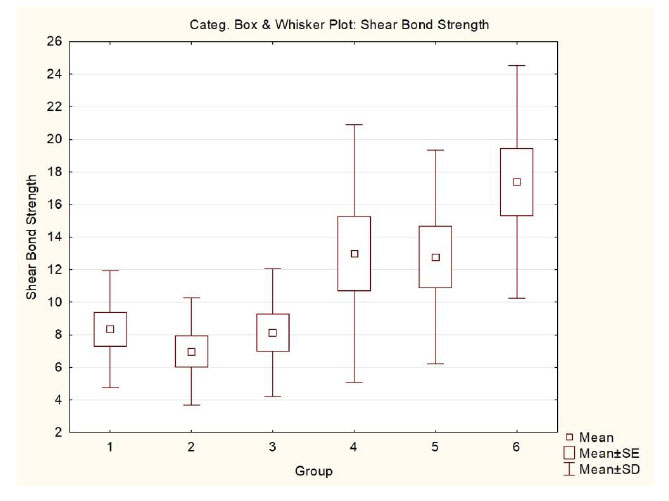
There was a statistically significant difference in G1, G2, and G3 in relation to G6 (p<0.001)(Table 2). Fig. (4) shows a graphical representation of the results obtained.
Regarding the ARI evaluation, there was a statistically significant difference between G1, G2, G3, and G4(p<0.001), where the predominance of ARI was score 3, suggesting the presence of much adhesive material in the enamel after debonding, as compared to G5 and G6, with a predominance of score 0, suggesting little or no adhesive material left in the enamel (Table 3). This indicates that the conventional composite presents greater adhesion to the bracket mesh base than the hydrophilic composite, which showed greater adhesion to the enamel.
| Effect | Univariate Tests of Significance for Shear Bond Strength Sigma-restricted parameterization Effective hypothesis decomposition |
||||
|---|---|---|---|---|---|
| SS | Degr. of Freedom |
MS | F | p | |
| Intercept | 6200.97 | 1 | 6200.97 | 183.09 | 0.000* |
| Composite | 245.52 | 1 | 245.52 | 7.24 | 0.008* |
| Contamination | 100.65 | 1 | 100.65 | 2.97 | 0.089 |
| pH cycling | 131.89 | 1 | 131.89 | 3.89 | 0.052 |
| Error | 2302.98 | 68 | 33.86 | ||
| Group | Composite | Contamin. | pH cycling |
Mean (±s.d.) |
Min. / Max. | Median |
|---|---|---|---|---|---|---|
| G1 | hydrophilic | Yes | Yes | 8.33 (±3.59) a |
3.23 / 14.21 | 7.76 |
| G2 | hydrophilic | Yes | No | 6.98 (±3.29) a |
0.30 / 13.76 | 6.78 |
| G3 | hydrophilic | No | Yes | 8.12 (±3.93) a |
3.55 / 16.52 | 7.53 |
| G4 | hydrophilic | No | No | 12.98 (±7.90) ab |
0.04 / 25.53 | 12.36 |
| G5 | conventional | No | Yes | 12.77 (±6.55) ab |
0.70 / 27.02 | 11.98 |
| G6 | conventional | No | No | 17.38 (±7.14) b |
5.52 / 33.55 | 18.08 |
| ARI Score (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Composite | Contamination | pH cycling | 0 | 1 | 2 | 3 | P |
| G1 | hydrophilic | Yes | Yes | 1 (8.3) | 3 (24.9) | 1 (8.3) | 7 (58.3) | <0.001 |
| G2 | hydrophilic | Yes | No | 0 (0.0) | 0 (0.0) | 1 (8.3) | 11 (91.6) | |
| G3 | hydrophilic | No | Yes | 0 (0.0) | 0 (0.0) | 2 (16.6) | 10 (83.3) | |
| G4 | hydrophilic | No | No | 3 (24.9) | 1 (8.3) | 1 (8.3) | 7 (58.3) | |
| G5 | conventional | No | Yes | 9 (74.7) | 0 (0.0) | 0 (0.0) | 3 (24.9) | |
| G6 | conventional | No | No | 7 (58.3) | 2 (16.6) | 0 (0.0) | 3 (24.9) | |
4. DISCUSSION
The debonding of brackets and tubes during orthodontic treatment can increase the cost and time of treatment. One of the causes of failure may be associated with the material used for bonding. Therefore, factors such as cost, practicality of the technique, ease of acquisition, and consistency in handling are considered when choosing the adhesive system for bonding.
In the present study, the conventional composite was not tested under saliva contamination because these types of hydrophobic composites were not conceived for bonding under contaminated conditions, and the results of shear bond strength would probably be lower. We aimed to test each composite as the manufacturer indicated and for what each one indicates. Despite the limitations of an in vitro design, it was possible to verify the resistance of the hydrophilic and conventional composites regarding the pH cycling, and, for the hydrophilic composite, regarding contamination with saliva, isolating interference factors, such as chewing and deleterious habits, simulating the oral environment with pH cycling, incubator, and artificial saliva.
The conventional composite used the Transbond XT, which is the orthodontic light-curing resin, considered the “gold standard” of adhesive resistance [7, 9, 13, 14, 24, 25]. The hydrophilic composite Transbond Plus Color Change is a light-curing adhesive system that, according to the manufacturer, provides reliable, protective, and moisture-tolerant bonding. It is presented in pink color, facilitating the removal of the excesses and after light-curing, color changes to the one similar to tooth enamel. It can be indicated for bonding of ceramic and metallic brackets, and first and second molar tubes, due to hydrophilia.
Some studies have shown that contamination by saliva or blood decreases the shear strength of brackets or tubes bonded with the conventional hydrophobic composite Transbond XT [8, 9, 24]. The use of the hydrophilic composite Transbond Plus Color Change associated with the Self Etching Primer, in an environment contaminated by saliva, provided adequate adhesive resistance for clinical use [14]. However, Ferreira et al. [26] found a decrease in shear bond strength in the presence of saliva contamination when using a hydrophilic composite, not showing adequate adhesion strength for clinical use.
The shear strength of brackets bonded with the hydrophilic composite Transbond Plus Color Change associated with the conventional Self Etching Primer was lower than the conventional hydrophobic composite Transbond XT, but still showing satisfactory adhesive strength results for its clinical application, especially with no contamination [14].
Despite the differences between the means of shear strength, all experimental groups showed values considered to be clinically effective. Reynolds and Fraunhofer [27] reported that the bracket bond strength varying from 5.6 to 6.8 MPa is sufficient for good clinical performance, and resisting orthodontic and masticatory forces. In the literature, there are no clear guidelines about shear force limits, but a good biomaterial should allow good adhesion in order to sustain masticatory forces, but bonding values should not be too strong to avoid substrate loss. Therefore, the ideal biomaterial should have bonding forces included in the interval of 5–50 MPa, even if these limits are mostly theoretical [28]. In the present report, all the values ranged inside this interval.
Depra et al. [24] demonstrated that saliva reduces the shear strength when brackets are bonded with a hydrophobic resin. However, the bond strength is not affected by saliva contamination when the brackets are bonded with an adhesive system with hydrophilic properties.
Acid solutions have a negative effect on dental tissues and also on dental materials. The acid environment can alter the hardness [29], roughness [30], fluorescence intensity [31], and flexural properties [32]of restorative frameworks. The present report also showed the negative influence of the acid environment on the adhesion of orthodontic brackets.
In the evaluation of the ARI, it should be considered that there are two adhesive interfaces in orthodontic brackets bonding; one exists between the tooth substrate and the primer and another between the primer and the composite. The hydrophilic composite groups showed a predominance of adhesive material in the enamel after shearing, while the conventional composite groups showed, predominantly, little or no adhesive material left into the enamel. Similar results were found in the study by Ekhlassi et al. [25], where the hydrophilic groups had 50% of the samples with the material fully adhered to the enamel, and only 25% of the conventional samples did not have material adhered to the enamel.
This shows that the conventional composite Transbond XT has greater adhesion to the bracket mesh compared to the hydrophilic Transbond Plus Color Change, which has greater adhesion to enamel. Lon et al. [14] obtained different results where the conventional composite without contamination showed fractures in the adhesive/bracket interface, the hydrophilic composite with and without salivary contamination and the conventional group with salivary contamination presented fractures in the tooth/adhesive interface, suggesting little adhesive remaining in the enamel.
In the groups in which the conventional composite was used, the highest fracture rate occurred at the enamel/adhesive interface, contrary to some results in the literature, which verified the tendency to remain more adhesive in the tooth as compared to the bracket base [33, 34].
Fractures at the adhesive/bracket interface or inside the adhesive, with the bonding material remaining adhered to the tooth, are favorable to avoid damage such as enamel cracks and fractures, as the residue can be removed more safely with specific high-speed drills [35].
Although no association was found with contamination and the shear bond strength, the conventional composite showed higher adhesive resistance. Therefore, it is up to the professional to know and choose which material should be used in each patient and clinical situation. It is important to highlight the care with contamination and hygiene guidance of orthodontic patients to prevent demineralization under and around brackets and possible cavitation due to dental caries.
Limitations of this study are the in vitro design using bovine teeth. Further in vivo studies are necessary to evaluate the clinical performance of hydrophilic composites under normal oral conditions.
CONCLUSION
Contamination by saliva did not decrease the shear bond strength of metal brackets bonded with the hydrophilic composite. There was no influence of pH cycling on the adhesion strength of the composites used.
The highest index of adhesive failure between the resin/bracket interface occurred with a hydrophilic composite, while the conventional composite showed a fracture in the resin/tooth interface.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Human Research Ethics Committee of Ingá University Center Uningá, Maringá, Brazil (n. 3394761).
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available from the corresponding author [K.M.S.F] upon request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


