All published articles of this journal are available on ScienceDirect.
The Safety and Efficacy of Pre- and Post-Medication for Postoperative Endo- dontic Pain: A Systematic Review and Network Meta-analysis
Abstract
Background:
Postoperative Endodontic Pain is a major concern for dentists and their patients, with pain having been reported to occur in 25%–40% of patients treated. Therefore, the aim of this systematic review and Network Meta-analysis (NMA) was to identify the safety and efficacy of pre- and post-medication for reducing postoperative endodontic pain.
Methods:
A literature search was performed in the SCOPUS, MEDLINE, and ScienceDirect, and Cochrane Central databases until December 2019 with no language restriction. Randomized controlled trials evaluating the efficacy of pre- or post-medications compared with other agents, placebo, or no treatment in adult patients who underwent endodontic surgery for postoperative pain were included. The mean difference of postoperative pain was measured using the Standardized Mean Difference (SMD) with its 95% confidence interval (95% CI).
Results:
This Systematic Review included 62 Articles. Of them, 50 studies were included in the NMA. Among all medications, corticosteroids were ranked as the best treatment for the reduction of postoperative pain at 6 and 12 hours with a significant reduction in postoperative pain scores [SMD= -1.18, 95% CI (-1.51: -0.85)] and [SMD= -1.39, 95% CI (-1.77: -1.02)], respectively. Cyclooxygenase-2 (COX-2) inhibitors were ranked as the best treatment for the reduction of postoperative pain at 8 and 24 hours with a significant reduction in postoperative pain scores [SMD= -2.86, 95% CI (-6.05: -1.66)] and [SMD= -1.27, 95% CI (-2.10: -0.43)], respectively. Non-steroidal anti-inflammatory drugs (NSAIDs) significantly reduced the postoperative pain scores in all durations. For postoperative pain at 6 hours, Indomethacin, Novafen, Naproxen, Prednisolone, Ketorolac, Betamethasone, Dexamethasone, Deflazacort, Rofecoxib, Piroxicam, and Ibuprofen significantly reduced the pain score when compared with a placebo. All of these drugs demonstrated a significant reduction at 12 hours except Ketorolac.
Conclusion:
The current evidence suggests that pre- and post-medication can reduce postoperative pain after nonsurgical root canal treatment. Corticosteroids and COX-2 inhibitors showed significant control of the pain up to 12 hours after administration. However, NSAIDs demonstrated a high efficacy from administration and until two days after treatment. Indomethacin, Novafen, prednisolone, and Naproxen were ranked first in most analyzed durations.
1. INTRODUCTION
Postoperative pain during root canal therapy is a major undesirable complication for dentists and their patients. Anxiety and fear of pain during root canal treatment are the main reasons that prevent patients from attending dental offices [1]. It was estimated that the prevalence of post-endodontic pain ranges from 3% to 58% [2-4]. This condition is linked with the exacerbation of inflammatory response and the activation of inflammatory mediators such as prostaglandins, which cause the periapical activation of sensitive nociceptors [5]. Preoperative and procedural factors such as intracanal medicaments, mechanical instrumentation, microbial effects, and chemical irritants may cause periradicular tissue injury, which in turn causes post-endodontic pain [5, 6]. Endodontic treatment consists of restoring the form and function of teeth and controlling symptoms that address the primary concern of the patient as well as long-term possible complications, such as chronic pain [7]. Therefore, it is highly important to manage discomfort during and after root canal treatment.
In this regard, many drugs have been used to relieve post-endodontic pain, such as Non-steroidal Anti-inflammatory Drugs (NSAIDs), corticosteroids, opioids, cyclooxygenase-2 enzymes (COX-2) inhibitors, and combinations of drugs [8]. Today, the most common types of pharmacological agents prescribed for pain relief in dentistry are NSAIDs and paracetamol (acetaminophen) [9]. NSAIDs decrease inflammation, inhibit cyclooxygenase enzymes, and prevent new prostaglandin molecules, but have no effect on circulating molecules [10]. Moreover, corticosteroids have demonstrated significant efficacy in dentistry pain management [11]. Many randomized control trials were conducted to evaluate the efficacy of various oral pre- and post-medications such as prednisolone [12], ibuprofen [13], lornoxicam [14], indomethacin [15], gabapentin [14], and celecoxib [16]. They reported that premedication is effective for postoperative pain after nonsurgical root canal treatment. However, the best pain-reducing agent is yet to be identified, as these drugs were not to be ranked regarding their efficacy. A recent network meta-analysis was conducted by Nagendrababu and his colleagues, who aimed to identify the most effective oral premedication in reducing pain in adults after nonsurgical root canal therapy [17]. Nevertheless, their study failed to include all available evidence, which eventually affected their conclusion. In this systematic review and network meta-analysis, we aimed to summarize current evidence on the efficacy of pre- and post-medication for the treatment of postoperative endodontic pain and rank the available drugs according to their efficacy.
2. METHODS
This systematic review and network meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for Network Meta-analyses of Health Care Interventions [18].
2.1. Search Strategy
A computerized search of Medline via PubMed, the Cochrane Library, Scopus, and Science direct was conducted using the following keywords “endodontic”, “root canal treatment”, “root canal therapy”, “NSAIDs”, “Non-Steroidal Anti Inflammatory Drugs”, “analgesics”, “paracetamol”, “Steroids”, “corticosteroids”, “Opioid”, “narcotic”, and “postoperative pain”. There was no language or publication date restriction. Additionally, the references of the retrieved trial were hand searched for further relevant articles.
2.2. Inclusion and Exclusion Criteria
We included studies that were eligible according to the following criteria:
(1) Population: studies that enrolled patients who presented with endodontic pain and received a diagnosis of pulpal pathosis necessitating initial nonsurgical endodontic treatment.
(2) Intervention: studies that used oral, intramuscular, supraperiosteal, intraligamentary injection, intracanal or systemic use of NSAIDs, corticosteroids, COX-2 inhibitors, or opioids.
(3) Comparison: placebo-controlled studies.
(4) Outcome: Management of postoperative pain assessed by Visual Analogue Scale (VAS).
(5) Study design: Randomized control trials.
Literature reviews, Opinion papers, systematic reviews, case reports, animal studies, preclinical studies, and clinical guidelines were excluded.
2.3. Study Selection
Eligibility screening was conducted in two steps, each by two independent reviewers: a) title and abstract screening for matching the inclusion criteria, and b) full-text screening for eligibility to meta-analysis. Disagreements were resolved upon the opinion of a third reviewer.
2.4. Data Extraction
Relevant data were abstracted using a standardized extraction form. The form consisted of
(1) Study characteristics (name of the first author, year, country, intervention groups, study sample size, and main findings),
(2) Participant characteristics (age, sex, and VAS score),
(3) Types of intervention and comparator(s) (i.e., drugs NSAIDs, COX-2, Opioids, and corticosteroids) and dosage,
(4) Outcome measures (i.e., the primary outcome: pain scores at different time intervals; immediately after treatment, 6, 8, 12, 24, and 48 hours). Missing information was obtained by contacting the authors of the study. When the means and standard deviations were not mentioned in the text of the published study, values were extracted from the graphs using WebPlotDigitizer (Ankit Rohatgi, Austin, TX, https://automeris.io/WebPlotDigitizer/).
All extracted data were cross-checked by two reviewers, and discrepancies were resolved by consensus.
2.5. Risk of Bias Assessment
The revised Cochrane Collaboration’s risk of Bias Assessment Tool (ROB) was used to assess the risk of bias among the included studies [19]. Studies were evaluated for bias and categorized as having low, unknown, or a high risk of bias. The overall quality of the study was based on the 5 domains evaluated for bias: randomization, deviation from intended interventions, missing outcome data, outcome measurement, and selection of results. The overall score was low bias when all five domains were scored as low bias. The presence of at least two concerns in one of the domains rendered the study as having some concerns in bias. A study was evaluated as having high bias when at least one domain was scored to have high bias.
2.6. Data Synthesis and Statistical Analysis
The Standardized Mean Differences (SMD) in postoperative pain scores were calculated as the summary measures in MA. We chose SMD because changes in pain intensity scores were reported by different scales in trials, and the SMD can compare pain intensity scores in a uniform manner. In the case where variance data were not reported as standard deviation, it was estimated with algebraic recalculations or various approximation methods. Means and standard deviations were calculated from the reported medians, ranges, or Confidence Intervals (CIs) when not available. The presence of heterogeneity among the selected studies warranted the use of a random-effects model for the calculation of weighted Mean Differences (MDs) and 95% CIs in MA. The heterogeneity between trials was evaluated using I2 statistics. Random effects NMA using a consistency model was applied to synthesize the available evidence by combining direct and indirect evidence from different studies.
The global inconsistency test using a fitting design-by-treatment model was used to identify the disagreement between the direct and indirect estimates as a measure of inconsistency. Frequentist method to rank treatments in network “netrank” function was used to rank the various interventions (the higher the P-score, the better the intervention). Moreover, the split direct and indirect evidence in network meta-analysis “netsplit” function was used. Publication bias was assessed using a comparison-adjusted funnel plot. All analyses were performed with R version 1.2.5019 (© 2009-2019 RStudio, Inc.) using the “netmeta” and “meta” packages for NMA [20].
3. RESULTS
3.1. Search Strategy Results
Our search retrieved 1512 unique citations. Following title and abstract screening, 107 full-text articles were retrieved and screened for eligibility. Of them, 45 articles were excluded, and 62 RCTs articles (n= 5412 patients) were included in the systematic review, and 50 articles were included in the final analysis. The flow diagram of study selection for our systematic review and meta-analysis is shown in PRISMA diagram (Fig. 1). A summary of included studies and baseline characteristics of the populations is shown in Table 1.
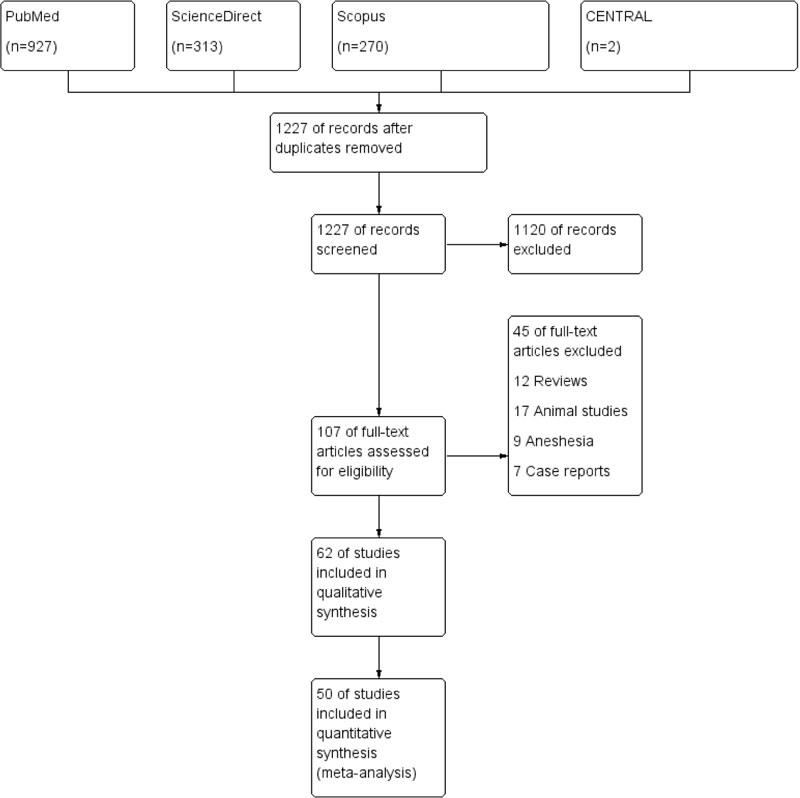
Table 1.
| Study | Year | Country | Sex (M,F) | Age | Treatment Groups | Dose (mg) | Sample Size | Follow-up Period (h) | VAS Scale | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Menke et al. [1] | 1999 | USA | 14,22 | >18 | Etodolac | 400 mg | 12 | 0, 4, 8, 12, 24, 48, 72 | 100 mm | Prophylactic ibuprofen significantly reduced post-endodontic pain at four and eight hours after initiation of treatment, when compared to etodolac and a placebo |
| Ibuprofen | 600 mg | 12 | ||||||||
| Placebo | 12 | |||||||||
| Gopikrishna and Parameswaran [2] | 2003 | India | 29, 16 | 18–64 | Ibuprofen | 600 mg | 15 | 4, 8, 12, 24, 48, 72 | 100 mm | Rofecoxib administration provides an effective reduction in post-endodontic pain |
| Rofecoxib | 50 mg | 15 | ||||||||
| Placebo | 15 | |||||||||
| Attar et al. [3] | 2008 | USA | 7, 7 | 44.9±4 | Ibuprofen tablets | 600 mg | 15 | 0, 6, 12, 18, 24 | 100 mm | Single-dose pretreatment analgesia alone in endodontic pain patients did not significantly reduce postoperative pain below the level of reduction in pain from endodontic treatment of ibuprofen 600 mg and the placebo group |
| 7, 6 | 41.6±4.3 | Ibuprofen liqui-gels | 600 mg | 15 | ||||||
| 9, 3 | 45.8±5.1 | Placebo | 15 | |||||||
| Saatchi et al. [4] | 2009 | Iran | NR | NR | Ibuprofen | 400 mg | 30 | 0, 2, 6, 10, 18, 36, 44, 54, 66, 72 |
0–10 | Diclofenac sodium continuous-release single dose pre-treatment of root canals compared to ibuprofen can prolong pain relief after root canal treatment for a longer period of time. |
| Diclofenac sodium | 100 mg | 30 | ||||||||
| Placebo | 30 | |||||||||
| Jalalzadeh et al. [5] | 2010 | Iran | 14, 6 | 18–59 | Prednisolone | 30 mg | 20 | 6, 12, 24 | 10 cm | Postendodontic pain was substantially reduced by preoperative administration of a single oral dose of prednisolone compared with placebo |
| 14, 6 | Placebo | 20 | ||||||||
| Arslan et al. [6] | 2011 | Turkey | 16, 32 | 18–52 | Tenoxicam | 20 mg | 16 | 6, 12, 24, 48, 72 | 100 mm | A prophylactic single dose of 20 mg tenoxicam or 200 mg Ibuprofen administration before RCT provides effective reduction of post-operative pain at 6 h |
| Ibuprofen | 200 mg | 16 | ||||||||
| Placebo | 16 | |||||||||
| Ashraf et al. [7] | 2013 | Iran | 7, 7 | 18-57 | celecoxib | 400mg | 15 | 4, 8, 12, 24, 48 | 170mm | Prophylactic Celecoxib is not recommended for post-endodontic pain reduction especially in cases with gastrointestinal (GI) problems |
| 8, 6 | Placebo | 15 | ||||||||
| Atbaei et al. [8] | 2010 | Iran | 36, 29 | 14-65 | piroxicam | 8mg | 35 | 4, 8, 12, 24, 48 | 10mm | Piroxicam is highly effective for reducing post-endodontic pain in vital teeth with irreversible pulpitis during the first 48 h. It was found to be much more effective than a similar lidocaine injection in reducing postoperative endodontic pain |
| Placebo | 30 | |||||||||
| Baradaran et al. [9] | 2014 | Iran | 26, 19 | 20-45 | ibuprofen | 400mg | 15 | 4, 6, 12, 24, 48, 72 | 10mm | Alprazolam may enhance the analgesic efficacy of ibuprofen in post-endodontic pain |
| Ibuprofen+alprazolam | 400mg+0.5mg | 15 | ||||||||
| Placebo | 15 | |||||||||
| Douglas [10] | 2004 | Portugal | 3,17 | 16-61 | Rofecoxib | 50mg | 20 | 4,8,10,12,24 | 10mm | Single dose of COX-2 inhibitors maybe sufficient to prevent post-endodontic pain |
| 4,16 | Diclofenac sodium | 50mg | 20 | |||||||
| 5,15 | Placebo | 20 | ||||||||
| Ehsani et al. [11] | 2012 | Iran | NA | NA | NAC | 400mg | 20 | 6, 8, 12, 24 | 10mm | The prophylactic ibuprofen and NAC failed to clearly reflect their effect on cytokines levels in exudates of chronic periapical lesions. On the other hand, it seems that NAC can be a substitute for ibuprofen in the management of post endodontic pain |
| Ibuprofen | 400mg | 20 | ||||||||
| NAC + Ibuprofen | 400 + 200mg | 20 | ||||||||
| placebo | 20 | |||||||||
| Elkhadem et al. [12] | 2017 | Egypt | 78, 122 | 18-35 | Prednisolone | 40mg | 200 | 6, 12, 24 | 100mm | A single dose of prednisolone was beneficial to control short-term post-obturation pain in patients with symptomatic irreversible pulpitis reducing pain incidence after 24 h by approximately 30% and postoperative analgesic intake by approximately 55% |
| 63, 137 | placebo | 200 | ||||||||
| Flath et al. [13] | 1987 | USA | 116, 4 | 20-80 | Placebo | 29 | 3, 7, 24 | 100mm | Endodontic treatment significantly reduced post-operative pain in preoperatively symptomatic patients. Doses of 100 or 200 mg of flurbiprofen resulted in minimal side effects | |
| Flurbiprofen | 100mg | 87 | ||||||||
| Isik et al. [14] | 2014 | Turkey | 7, 23 | 18-45 | Gabapentin | 600mg | 30 | 4, 8, 12, 24 | 100mm | Prophylactic lornoxicam controlled post-endodontic treatment pain more effectively than did the placebo drugs, and gabapentin was more effective in controlling the pain than either lornoxicam or the placebo. |
| lornoxicam | 8mg | 30 | ||||||||
| placebo | 30 | |||||||||
| Joshi et al. [15] |
2016 | India | 11, 11 | 18-65 | piroxicam | 40mg | 22 | 4, 8, 12, 24, 48 | 10 cm | Peroxicam group perceived less post-endodontic pain as compared to placebo at all the time intervals |
| 12, 10 | Placebo | 22 | ||||||||
| Kaviani et al. [16] | 2011 | Iran | NA | 15-45 | Ketamine | 10mg | 18 | 24 | 10mm | A low dose of ketamine might be beneficial for enhancing the effect of local anesthetics |
| Placebo | 18 | |||||||||
| Khorasani et al. [17] | 2011 | Iran | 8, 8 | 25-50 | ibuprofen | 400mg | 16 | 6, 12, 24, 48, 72 | 100mm | Prophylactic use of Ibuprofen and sulindac for reduction of post-endodontic pain is not suggested |
| 9, 7 | sulindac | 200mg | 16 | |||||||
| 6, 10 | placebo | 16 | ||||||||
| Mehrvarzfar et al. [18] | 2016 | Iran | 9, 11 | 32+4.6 | placebo | 20 | 6, 12, 24 | 170mm | Pretreatment PDL injection of dexamethasone can significantly reduce the post-treatment endodontic pain in patients with symptomatic irreversible pulpitis. | |
| 10, 10 | 26.1+9.8 | lidocaine | 0.2ml | 20 | ||||||
| 8, 12 | 30.3+4.2 | dexamethasone | 8 mg | 20 | ||||||
| Mehrvarzfar et al. [19] | 2012 | Iran | 15, 9 | 31.4+10.7 | placebo | 24 | 6, 12, 24 | 10mm | A single oral dose of Naproxen, Novafen and Tramadol taken immediately after treatment reduced postoperative pain following pulpectomy and root canal preparation of teeth with irreversible pulpitis. | |
| 13, 11 | 29.5+6.9 | tramadol | 100mg | 24 | ||||||
| 11, 12 | 29.6+8.1 | Novafen | 325 mg of paracetamol, 200 mg ibuprofen and 40 mg caffeine anhydrous) |
23 | ||||||
| 14, 10 | 28.4+7.6 | naproxen | 500mg | 24 | ||||||
| Menhinick et al. [20] | 2004 | USA | 8, 11 | 24-80 | placebo | 19 | 4, 8 | 100mm | The results demonstrate that the combination of ibuprofen with acetaminophen may be more effective than ibuprofen alone for the management of postoperative endodontic pain. | |
| 6, 14 | 21-61 | ibuprofen | 600 | 20 | ||||||
| 2, 16 | 19-58 | ibuprofen + paracetamol | 600mg + 1000mg | 18 | ||||||
| Mirzaie et al. [21] | 2011 | Iran | 56, 34 | 18-65 | Celecoxib | 200mg | 30 | 4, 8, 12, 24, 48 | 100 mm | Use of Gelofen or Celecoxib before treatment reduces post-endodontic pain. These drugs can be prescribed before initiation of treatment as effective agents for the reduction of post-endodontic pain. |
| Gelofen | 400mg | 30 | ||||||||
| Placebo | 30 | |||||||||
| Mokhtari et al. [22] | 2016 | Turkey | 9, 13 | 19-0 | Ibuprofen | 400mg | 22 | 8, 12, 24 | 100mm | Premedication with ibuprofen and indomethacin can effectively control short term post-operative pain; the lower incidence of side effects and greater analgesic power of ibuprofen make it a superior choice. |
| 7, 15 | Indomethacin | 25mg | 22 | |||||||
| 13, 9 | Placebo | 22 | ||||||||
| Negm 1st group [23] | 1989 | Egypt | NA | 16-71 | Piroxicam | 20mg | 48 | 2, 4, 8 | 1 to 4 | Piroxicam was more effective than diclofenac or the placebo. Diclofenac required a longer time to reach maximum effectiveness. Piroxicam’s superiority was greater at the first and second days after the initial dose of medication was taken. |
| Diclofenac sodium | 50mg | 52 | ||||||||
| Placebo | 43 | |||||||||
| Negm 2nd group [23] | Piroxicam | 20mg | 45 | |||||||
| diclofenac sodium | 50mg | 40 | ||||||||
| placebo | 40 | |||||||||
| Negm 1st group [24] | 1994 | Egypt | NA | 18-78 | diclofenac | 75mg | 65^ | 2, 4, 8, 12 | 1 to 4 | Post-endodontic pain occurred with less frequency when the teeth were treated with diclofenac, but diclofenac-treated and ketoprofen-treated cases were not significantly different in controlling post-endodontic pain. An increase in the number of patients who reported a complete absence of pain was recorded when hyaluronidase was added to the study medications. However, the difference between the medications and medication-hyaluronidase was not of statistical significance. |
| diclofenac-hyaluronidase | 75mg + 1500 iu | 63^ | ||||||||
| Ketoprofen | 100mg | 60^ | ||||||||
| Ketoprofen-hyaluronidase | 100mg + 1500 iu | 70^ | ||||||||
| Placebo | 58^ | |||||||||
| Placebo-hyaluronidase | 1500 iu | 51^ | ||||||||
| Negm 2nd group [24] | diclofenac | 75mg | 73^ | |||||||
| diclofenac-hyaluronidase | 75mg + 1500 iu | 70^ | ||||||||
| Ketoprofen | 100mg | 66^ | ||||||||
| Ketoprofen-hyaluronidase | 100mg + 1500 iu | 60^ | ||||||||
| Placebo | 60^ | |||||||||
| Placebo-hyaluronidase | 1500 iu | 64^ | ||||||||
| Nekoofar et al. [25] | 2003 | USA | NA | >15 | meloxicam | 15mg | 17 | 8, 24 | 9cm | Based on the two-way repeated measures ANOVA, the reduction in pain with meloxicam, piroxicam, and placebo was not significantly different (p=0.058), although the mean change of pain was greater with meloxicam over piroxicam and greater with piroxicam than placebo. |
| piroxicam | 20mg | 17 | ||||||||
| placebo | 17 | |||||||||
| Praveen et al. [26] | 2017 | India | 15, 14 | 18-50 | Ketorolac | 20mg | 31 | 0, 6, 12, 24, 48 | 10 cm | Single pre-treatment dose of prednisolone has a more sustained effect in reducing post-endodontic pain compared with placebo or ketorolac. |
| 16, 14 | prednisolone | 30mg | 31 | |||||||
| 13, 14 | placebo | 31 | ||||||||
| Ramazani et al. [27] | 2013 | Iran | 15, 12 | 18-65 | ibuprofen | 400mg | 30 | 4, 8, 12, 24, 48, 72 | 100mm | The obtained results of the trial revealed that prophylactic use of 2 g Zintoma is not an effective pain-relieving agent. |
| 13, 11 | zintoma | 2000mg | 30 | |||||||
| 10, 11 | placebo | 30 | ||||||||
| Rashka et al. [28] | 2013 | India | NA | NA | diclofenac sodium | 30mg | 26 | 4, 8, 12, 24, 48 | 10mm | Diclofenac Sodium was found to be highly effective in reducing post-endodontic pain of vital teeth with irreversible pulpitis during the first 48 h. |
| placebo | 26 | |||||||||
| Ryan et al. [29] | 2008 | USA | 6, 8 | NA | placebo | 14 | 0, 6, 12, 18, 24 | NA | Statistical analysis of the data showed that ibuprofen 600 mg provided statistically significantly greater analgesic effect than placebo at 6 and 12 hours (P=0.0014 and 0.0024), and pentazocine/naloxone provided statistically significantly greater analgesic effect than placebo at 12 hours (P =0.0084). | |
| 8, 7 | ibuprofen | 600mg | 15 | |||||||
| 6, 8 | talwin | 50mg | 14 | |||||||
| Salarpoor et al. [30] | 2013 | Iran | 6, 13 | 31.3 | ibuprofen | 400mg | 19 | 6, 12, 24, 48 | 10mm | The results demonstrate that betamethasone and indomethacin may be more effective than ibuprofen for the management of post-operative pain after nonsurgical endodontic treatment when patients present with moderate to severe pain |
| 4, 17 | 24.5 | betamethasone | 2mg | 21 | ||||||
| 7, 15 | 28 | indomethacin | 75mg | 22 | ||||||
| 6, 14 | 29 | placebo | 20 | |||||||
| Sethi et al. [31] | 2014 | India | 12, 6 | 18-60 | Tapentadol | 100mg | 20 | 0, 6, 12, 18, 24 | 10cm | Single oral dose of 10 mg of ketorolac and 100mg of tapentadol as a pretreatment analgesic significantly reduced postoperative endodontic pain in patients with symptomatic irreversible pulpitis when compared to 400 mg of etodolac |
| Etodolac | 400mg | 20 | ||||||||
| Ketorolac | 10mg | 20 | ||||||||
| Elzaki et al. [32] | 2016 | Sudan | 66, 104 | 33+10.5 | paracetamol | 1000mg | 34 | 1, 2, 3, 4, 6, 8 | NA | The combination of ibuprofen/paracetamol, taken immediately after initial endodontic therapy and root canal preparation in teeth with irreversible pulpitis, reduced post-endodontic pain |
| Ibuprofen + paracetamol | 600 + 1000mg | 33 | ||||||||
| Mefenamic acid + paracetamol | 500mg + 1000mg | 34 | ||||||||
| Diclofenac K + paracetamol | 50mg + 1000mg | 35 | ||||||||
| Placebo | 34 | |||||||||
| Jorge-Araújo et al. [33] | 2018 | Brazil | 7, 12 | 18-66 | Placebo | 20 | 4, 8, 12, 24, 48 | NA | Preoperative administration of Ibuprofen or dexamethasone reduces post-endodontic pain and discomfort in comparison with a placebo. Premedication with anti-inflammatory drugs could contribute to control of the post-endodontic pain, mainly in patients more sensitive towards pain | |
| 7, 12 | Ibuprofen | 400mg | 20 | |||||||
| 7, 11 | Dexamethasone | 8mg | 20 | |||||||
| Jenarthanan et al. [34] | 2018 | India | 7,3 | 30±6 | Oral diclofenac sodium | 75mg | 10 | 6,12,24,48 | 10cm | In patients with low pain threshold, intra-ligamentary route of administration is effective in controlling pain of endodontic origin postoperatively. |
| 5,5 | 26±9 | Intraligamentary route of diclofenac sodium | NA | 10 | ||||||
| 6,4 | 28±7 | Placebo | 10 | |||||||
| Yavari et al. [35] | 2019 | Iran | NA | 20-50 | Placebo | 64 | 6, 12, 24, 48, 72 | 0-10 | Infiltration of long-acting betamethasone and dexamethasone resulted in decreased postoperative pain experience. Dexamethasone was more effective in alleviating pain within the first 24-hour period after treatment. Infiltration of long-acting betamethasone and dexamethasone exhibited the same efficacy in 48 hours. The efficacy of long-acting betamethasone in pain relief lasted for 7 days. The QOL in the 2 groups receiving corticosteroids was higher than that in the placebo group. | |
| Betamethasone | 0.7 mL | 66 | ||||||||
| Dexamethasone | 4mg | 64 | ||||||||
| Makkar et al. [36] | 2012 | India | 7,3 | 39.6 yrs | Ibuprofen and paracetamol | 400 mg,325 mg | 10 | 6,12,24 | 10 cm | A single oral dose of diclofenac sodium and paracetamol and ibuprofen and paracetamol combination reduced postoperative pain following pulpectomy and root canal preparation of teeth with irreversible pulpitis. |
| 6,4 | 41.3 yrs | Diclofenac sodium and paracetamol | 50 mg, 500mg | 10 | ||||||
| 6,4 | 37.9 yrs | Placebo | 10 | |||||||
| Doroschak et al. [37] | 1999 | USA | NA | 18-65 | Tramadol | 100 mg | 12 | 1,2,3 | 100 mm | NSAID/opiate combination, together with endodontic therapy, may be useful in the management of endodontic pain. |
| Flurbiprofen | 100 mg | 12 | ||||||||
| Tramadol/Flurbiprofen | 100 mg | 13 | ||||||||
| Placebo | 12 | |||||||||
| Konagala et al. [38] | 2019 | India | 62,70 | 18-50 | Piroxicam | 20 mg | 30 | 6,12,24,48,72 | 100 mm | Preoperative single oral dose of piroxicam or dexamethasone or deflazacort is equally effective in controlling post-endodontic pain. |
| dexamethasone | 4 mg | 30 | ||||||||
| deflazacort | 30 mg | 30 | ||||||||
| Placebo | 30 | |||||||||
| Ashraf [39] | 2002 | Iran | NA | NA | Rofecoxib | NA | 60 | 12 | 100mm | NA |
| Ibuprofen | ||||||||||
| Placebo | ||||||||||
| Chance et al. [40] | 1987 | USA | NA | NA | prednisolone | NA | 158 | NA | NA | The corticosteroid was effective in significantly reducing the incidence of postoperative pain in teeth where vital pulp was present. |
| Placebo | 142 | |||||||||
| Glassman et al. [41] | 1989 | USA | NA | NA | Dexamethasone | 4 mg | 19 | NA | NA | oral dexamethasone is sufficient to significantly reduce endodontic interappointment pain for teeth with asymptomatic vital-inflamed pulps. |
| Placebo | 18 | |||||||||
| Kaufman et al. [42] | 1994 | Israel | 16,29 | 19-71 | Methylprednisolone | 8 mg | 18 | 24 | NA | The tested drug significantly reduced the frequency and intensity of postoperative pain sequelae in the experimental set-up. |
| Mepivacaine | NA | 17 | ||||||||
| Placebo | NA | 10 | ||||||||
| Krasner et al. [43] | 1986 | USA | NA | NA | Dexamethasone | 5.25mg | 25 | 8,24 | 100 mm | Post-treatment endodontic pain was substantially reduced by administration of oral dexamethasone. The risks to the otherwise healthy patient seem to be minimal and acceptable |
| Placebo | 25 | |||||||||
| Liesinger et al. [44] | 1993 | USA | NA | NA | Dexamethasone | 8 mg | 106 | 1,4,8,24,48,72 | 9 cm | Patients who received dexamethasone took significantly fewer posttreatment pain medications than those who received the placebo |
| Placebo | ||||||||||
| Marshall et al. [45] | 1984 | USA | NA | NA | Dexamethasone | 4 mg | 50 | 4,24 | NA | Injection of the steroid (dexamethasone, 4 mg) significantly reduced both the incidence and severity of pain at 4 h post-treatment and reduced pain at 24 h post-treatment. |
| Placebo | ||||||||||
| Mehrvarzfar et al. [46] | 2008 | Iran | 34,66 | 21-58 | Dexamethasone | 4 mg | 50 | 6,12,24,48 | NA | Dexamethasone was considerably effective in controlling the severity of pain during the first 24 h; in contrast, there was no difference between dexamethasone and placebo groups 48 h after the first appointment. |
| Placebo | 50 | |||||||||
| Pochapski et al. [47] | 2009 | Brazil | 26,24 | 18-67 | Dexamethasone | 4 mg | 25 | 4,6,12,24 | NA | Preoperative single oral dose of dexamethasone substantially reduced post-endodontic pain |
| Placebo | 23 | |||||||||
| Rogers et al. [48] | 1999 | USA | NA | NA | Dexamethasone | 4mg | 12 | 6,12,24,48 | 100 mm | At the 12-h period, both dexamethasone and ketorolac provided statistically significant better pain relief than placebo. At the 24-h period, only ketorolac demonstrated better pain relief than the placebo. There were no statistically significant differences among the groups at 6 and 48 h. |
| Ketorolac tromethamine | 30 mg | 12 | ||||||||
| Ibuprofen | 600 mg | 12 | ||||||||
| placebo | 12 | |||||||||
| Shantiaee et al. [49] | 2012 | Iran | 30,60 | 18-42 | Dexamethasone | 4 mg | 30 | 4,8,24,48 | 9cm | Periapical infiltration of dexamethasone and morphine led to a considerable decrease in postoperative endodontic pain during the first 24 h after operation. Dexamethasone was more effective than morphine in pain reduction. |
| Morphine | 1 mg | 30 | ||||||||
| Placebo | 30 | |||||||||
| Zarrabi | 2003 | Iran | NA | NA | betamethasone | 4 mg | 50 | 6,12,24 | NA | NA |
| Placebo | 50 | |||||||||
| Zarrabi et al. [50] | 2007 | Iran | NA | NA | betamethasone | 2 mg | 20 | 6,12,24 | NA | NA |
| Placebo | 20 | |||||||||
| Sharma et al. [51] | 2015 | India | NA | NA | dexamethasone | 4 mg | 20 | 6,12,24 | 100 mm | NSAID resulted in significantly less post-operative endodontic pain at all time-intervals. Preoperative oral administration of Dexamethasone performed best in reducing pain post operatively. |
| Placebo | 20 | |||||||||
| Eftekhar et al. [52] | 2013 | NA | NA | NA | Triamcinolone | 1 mg | 40 | NA | NA | NA |
| Placebo | 40 | |||||||||
| Moradi et al. [53] | 2013 | Iran | NA | NA | dexamethasone | 4 mg | 15 | 6,12,24,48 | 10 cm | Administration of dexamethasone did not reduce post-operative pain severity in the first 12hours after endodontic treatment |
| Placebo | 15 | |||||||||
| Ahangari | 2009 | Iran | NA | NA | dexamethasone | 0.5 mg | 20 | 6,12,24 | 10 cm | NA |
| Placebo | 20 | |||||||||
| Fava [54] | 1998 | NA | NA | 28-64 | Otosporin | NA | 30 | 48 h/1 w | NA | No difference was observed in the incidence of post-operative pain between the two groups. |
| Placebo | 30 | |||||||||
| Ehrmann et al. [55] | 2003 | Australia | NA | NA | Triamcinolone acetonide | 58 | 4,24,48,72 | 100 mm | Ledermix is an effective intracanal medicament for the control of postoperative pain associated with acute apical periodontitis, with a rapid onset of pain reduction. | |
| Placebo | 71 | |||||||||
| Negm et al. [56] | 2001 | Egypt | NA | 15-75 | Kenacomb | NA | 245 | 24 | 100 mm | intracanal use of corticosteroid-antibiotic combination for controlling posttreatment endodontic pain. |
| Placebo | 230 | |||||||||
| Wells et al. [57] | 2011 | USA | 17,16 | 34.3±14.0 | Ibuprofen/acetaminophen | 600 mg/1000 mg | 35 | 24,48,72 | 100 mm | There were decreases in pain levels and analgesic use over time in the ibuprofen and ibuprofen/acetaminophen groups. |
| 20,15 | 37.3± 14.7 | Ibuprofen | 600 mg | 36 | ||||||
| Battrum et al. [58] | 1996 | USA | NA | NA | Ketorolac | 10 mg | 10 | 6,24 | 100mm | There was no significant difference in pain relief between the two groups treated with different drug regimens |
| Placebo | 10 | |||||||||
| Torabinejad et al. [59] | 1994 | NA | NA | NA | Salicylic acid | 650 mg | 50 | 30, 36, 42, 48, 54, 60, 66, 72 | 90mm | Ibuprofen, ketoprofen, erythromycin base, penicillin, and methylprednisolone plus penicillin were more effective than placebo within the first 48 h following complete instrumentation. |
| Acetaminophen | 650 mg | 57 | ||||||||
| Ibuprofen | 400 mg | 57 | ||||||||
| Ketoprofen 50 mg | 50 mg | 53 | ||||||||
| Acetaminophen + codeine | 325 mg/60 mg | 48 | ||||||||
| Moskow et al. [60] | 1984 | NA | NA | NA | Dexamethasone | 4 mg | 26 | 24,48,72 | 100 mm | A statistically significant decreased incidence of pain was reported for the corticosteroid cases as compared to the control at the 24-hour time period (p<0.05) |
| Placebo | 24 |
3.2. Characteristics and Quality of the Included Studies
A total of 5412 patients, including males and females between the ages of 15 and 80 years, from the included studies, formed the sample size for the NMA. The origin countries of included studies were Iran (n=21), USA (n=15), India (n=9), Egypt (n=3), Turkey (n=3), Brazil (n=2), Israel (n=1), Portugal (n=1), Sudan (n=1), and Australia (n=1), and four studies were found to be non-reported. Negm study consists of two trials; therefore, each one is considered as a separate study. The quality of the 62 included studies is described in Table 2. Thirty-seven studies had a low risk of bias, 10 studies had a high risk of bias, and 15 studies had some concerns.
| Study | Year | Randomization | Allocation Concealment | Blinding of Participants and Personnel | blinding of Outcome Assessors | Attrition Bias | Selection Bias | Other Bias | Overall |
|---|---|---|---|---|---|---|---|---|---|
| Arslan et al. | 2011 | low | unclear | low | low | low | low | low | Low |
| Ashraf et al. | 2013 | low | unclear | low | low | low | low | low | Low |
| Atbaei et al. | 2010 | low | unclear | low | unclear | low | low | low | Some concerns |
| Attar et al. | 2008 | low | unclear | low | low | low | low | low | Low |
| Baradaran | 2014 | low | unclear | low | low | low | low | low | Low |
| Douglas | 2004 | low | unclear | low | low | low | low | low | Low |
| Ehsani | 2012 | low | unclear | low | low | low | low | low | Low |
| Elkhadem | 2017 | low | low | low | low | low | low | low | Low |
| Elzaki | 2016 | low | unclear | low | low | low | low | low | Low |
| Flath | 1987 | low | unclear | low | low | low | low | low | Low |
| Gopikrishna and Parameswaran | 2003 | low | unclear | low | low | low | low | low | Low |
| Isik | 2014 | low | unclear | low | low | low | low | low | Low |
| Jalalzadeh et al. | 2010 | low | unclear | low | low | low | low | low | Low |
| Jorge-Araújo | 2018 | low | low | low | low | low | low | low | Low |
| Joshi | 2016 | low | unclear | low | low | low | low | low | Low |
| Kaviani | 2011 | low | unclear | low | low | low | low | low | Low |
| Khorasani | 2011 | low | unclear | low | low | low | low | low | Low |
| Mehrvarzfar | 2012 | low | unclear | low | low | low | low | low | Low |
| Mehrvarzfar | 2016 | low | unclear | low | low | low | low | low | Low |
| Menhinick | 2004 | low | unclear | low | low | low | low | low | Low |
| Menke et al. | 1999 | low | unclear | low | unclear | low | low | low | Some concerns |
| Mirzaie | 2011 | low | unclear | low | low | low | low | low | Low |
| mokhtari | 2016 | low | unclear | low | low | low | low | low | Low |
| Negm | 1989 | low | unclear | low | unclear | low | low | low | Some concerns |
| Negm | 1994 | low | unclear | low | unclear | low | low | low | Some concerns |
| Nekoofar | 2003 | low | unclear | low | low | low | low | low | Low |
| Praveen | 2017 | low | low | low | low | low | low | low | Low |
| Ramazani | 2013 | low | unclear | low | low | low | low | low | Low |
| Rashka | 2013 | low | unclear | unclear | low | low | low | low | Some concerns |
| Ryan | 2008 | low | unclear | low | low | low | low | low | Low |
| Saatchi et al. | 2009 | low | unclear | low | low | low | low | low | Low |
| Salarpoor | 2013 | low | unclear | low | low | low | low | low | Low |
| Sethi | 2014 | low | unclear | low | low | low | low | low | Low |
| Yavari | 2019 | low | low | low | low | low | low | low | Low |
| Makkar | 2012 | low | unclear | low | low | low | low | low | Low |
| Doroschak | 1999 | low | unclear | low | low | low | low | low | Low |
| Konagala | 2019 | low | low | low | low | low | low | unclear | Low |
| Jenarthanan | 2018 | low | unclear | unclear | unclear | low | low | low | Some concerns |
| Ashraf et al. | 2002 | low | unclear | low | low | low | low | low | Low |
| Chance | 1987 | unclear | low | unclear | low | low | unclear | unclear | Some concerns |
| Glassman | 1989 | unclear | low | unclear | unclear | low | unclear | unclear | Some concerns |
| Kaufman | 1994 | low | unclear | unclear | unclear | low | unclear | unclear | Some concerns |
| Krasner | 1986 | low | low | low | unclear | low | low | unclear | Some concerns |
| Liesinger | 1993 | unclear | unclear | low | low | low | low | low | Some concerns |
| Marshall | 1984 | low | unclear | low | low | low | low | low | Low |
| Mehrvarzfar et al. | 2008 | low | unclear | low | low | low | unclear | low | Some concerns |
| Pochapski | 2009 | low | unclear | low | low | low | unclear | low | Some concerns |
| Rogers | 1999 | low | unclear | unclear | unclear | low | low | low | Some concerns |
| Shantiaee | 2012 | low | unclear | low | low | low | low | low | Low |
| Zarrabi | 2003 | low | unclear | low | high | low | low | high | High |
| Zarrabi | 2007 | low | unclear | low | low | high | low | low | High |
| Sharma | 2015 | low | unclear | low | low | high | low | low | High |
| Eftekhar | 2013 | low | unclear | low | low | high | low | low | High |
| Moradi | 2013 | low | unclear | low | low | high | low | low | High |
| Ahangari | 2009 | low | unclear | low | low | high | unclear | low | High |
| Fava | 1998 | low | unclear | high | high | high | unclear | low | High |
| Ehrmann | 2003 | unclear | unclear | unclear | unclear | low | low | low | Some concerns |
| Negm | 2001 | low | low | low | low | low | low | low | Low |
| Wells | 2011 | low | unclear | low | low | low | low | low | Low |
| Battrum | 1996 | unclear | unclear | high | high | high | low | low | High |
| Torabinejad | 1994 | high | high | low | unclear | low | low | low | High |
| Moskow | 1984 | low | high | high | unclear | low | low | low | High |
3.3. Effects on the Primary Outcomes
3.3.1. Postoperative Pain for Treatment Intervention Categorized by Pharmacologic Group
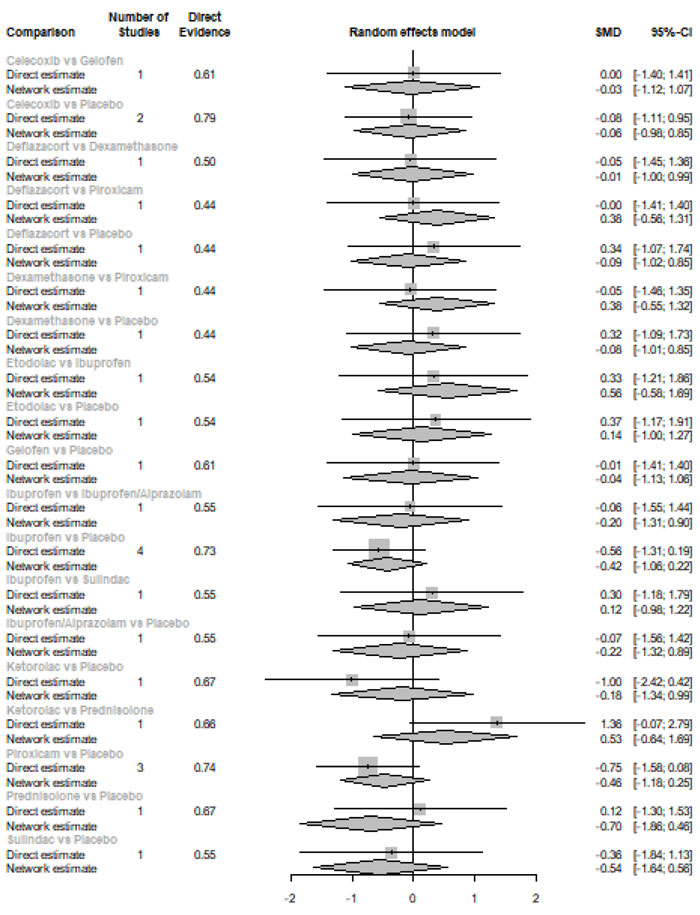
Immediately after procedure: Among all medications, opioids were ranked as the best treatment for the reduction of postoperative pain [SMD= -1.16, 95% CI (-1.96: -0.36), P-score= 0.91]. Moreover, NSAIDs showed a significant reduction in pain after endodontic treatment [SMD= -0.63, 95% CI (-0.89: -0.36), P-score= 0.61]. On the other hand, there was no significant difference between corticosteroids, COX-2 inhibitors, and placebo in this period. Pooled analysis was heterogeneous (Q=373.01; I2=84.7%; P<0.0001) due to the significant variation among the analyzed categories (Fig. 2a). Publication bias analysis showed that there was no detected bias according to the Egger test (p=0.07). Split analysis demonstrated that there was no significant difference between corticosteroid vs. placebo or NSAIDs (Appendix Fig. 1). Network ranking graph showed the rank of categories immediately after the procedure (Fig. 3a). League table is presented in Appendix Table 1.
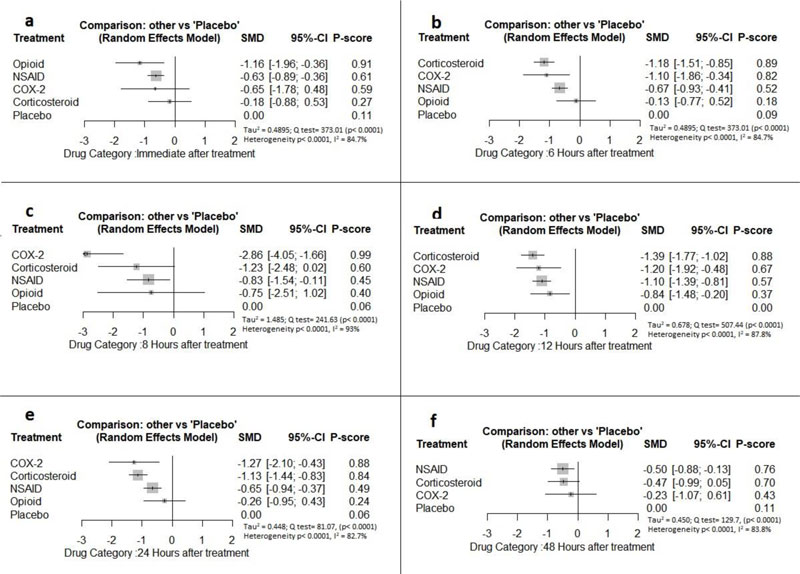
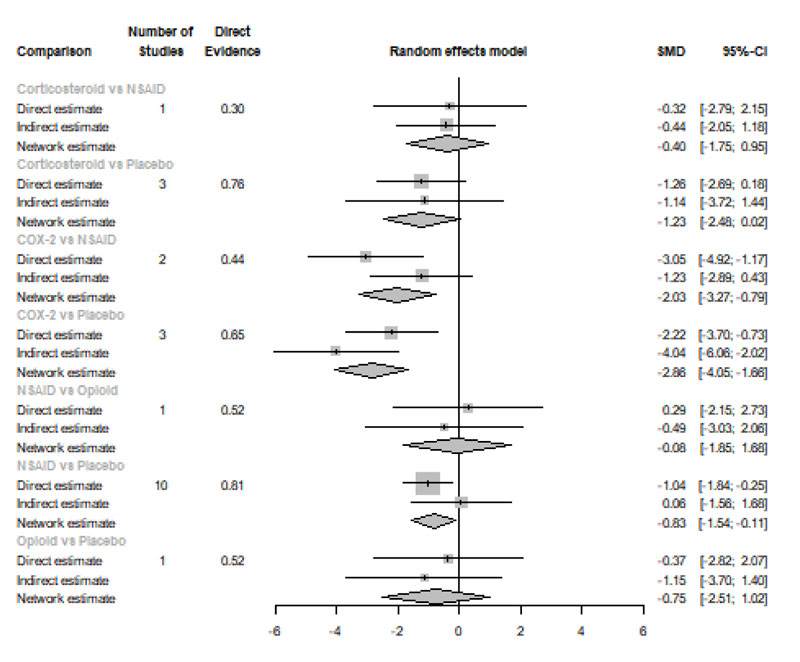
Six Hours after Procedure: Interestingly, the efficacy of corticosteroids dramatically increased, reaching the first rank in terms of the best treatment for the reduction of postoperative pain [SMD= -1.18, 95% CI (-1.51: -0.85), P-score= 0.89], and the efficacy of opioids dramatically decreased, scoring the fourth rank [SMD= -0.13, 95% CI (-0.77: 0.52), P-score= 0.18]. NSAIDs showed a significant reduction in pain after endodontic treatment [SMD= -0.67, 95% CI (-0.93: -0.41), P-score= 0.52]; however, it scored the third rank after the COX-2 inhibitors [SMD= -1.10, 95% CI (-1.86: -0.34), P-score= 0.82]. Pooled analysis was heterogeneous (Q=373.01; I2=84.7%; P<0.0001) due to the significant variation among the analyzed categories (Fig. 2b). Publication bias analysis showed a detected bias according to the Egger test (p=0.005). Split analysis demonstrated no significant difference between NSAIDs vs. COX-2 inhibitors or vs. Opioids (Appendix Fig. 2). Network ranking graph showed the rank of categories at 6 hours after the procedure (Fig. 3b). League table is presented in Appendix Table 2.
Eight Hours after Procedure: at this period, only COX-2 inhibitors and NSAIDs showed a significant effect in reducing the postoperative pain [SMD= -2.86, 95% CI (-4.05:-1.66), P-score= 0.99] and [SMD= -0.83, 95% CI (-1.54:-0.11), P-score= 0.45], respectively. Pooled analysis was heterogeneous (Q=241.63; I2=93%; P<0.0001) due to the significant variation among the analyzed categories (Fig. 2c). Publication bias analysis showed that there was no detected bias according to the Egger test (p=0.60). Split analysis demonstrated no significant difference between NSAIDs vs. corticosteroids or vs. Opioids (Appendix Fig. 3). Network ranking graph showed the rank of categories at 8 hours after the procedure (Fig. 3c). League table is presented in Appendix Table 3.
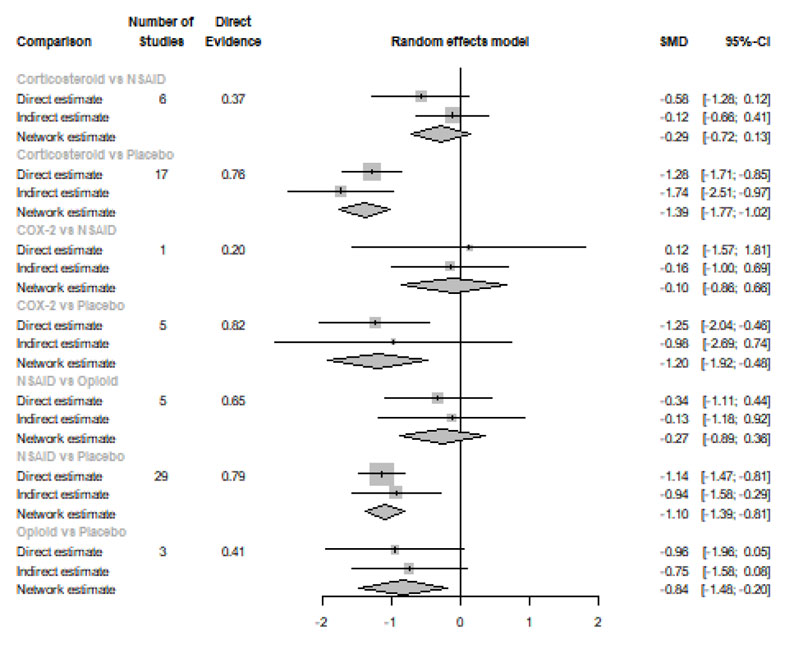
Twelve Hours after Procedure: All medication showed a signіficant reduction when compared to placebo; Corticos- teroids (SMD= -1.39), COX-2 inhibitors (SMD= -1.20), NSAIDs (SMD= -1.10), and Opioids (SMD= -0.84). Pooled analysis was heterogeneous (Q=507.44; I2=87.8%; P<0.0001) due to the significant variation among the analyzed categories (Fig. 2d). Publication bias analysis showed that there was a detected bias according to the Egger test (p=0.0001). Split analysis demonstrated that there was no significant difference between NSAIDs vs. corticosteroids, Opioids, and COX-2 inhibitors (Appendix Fig. 4). Network ranking graph showed the rank of categories at 12 hours after the procedure (Fig. 3d). League table is presented in Appendix Table 4.
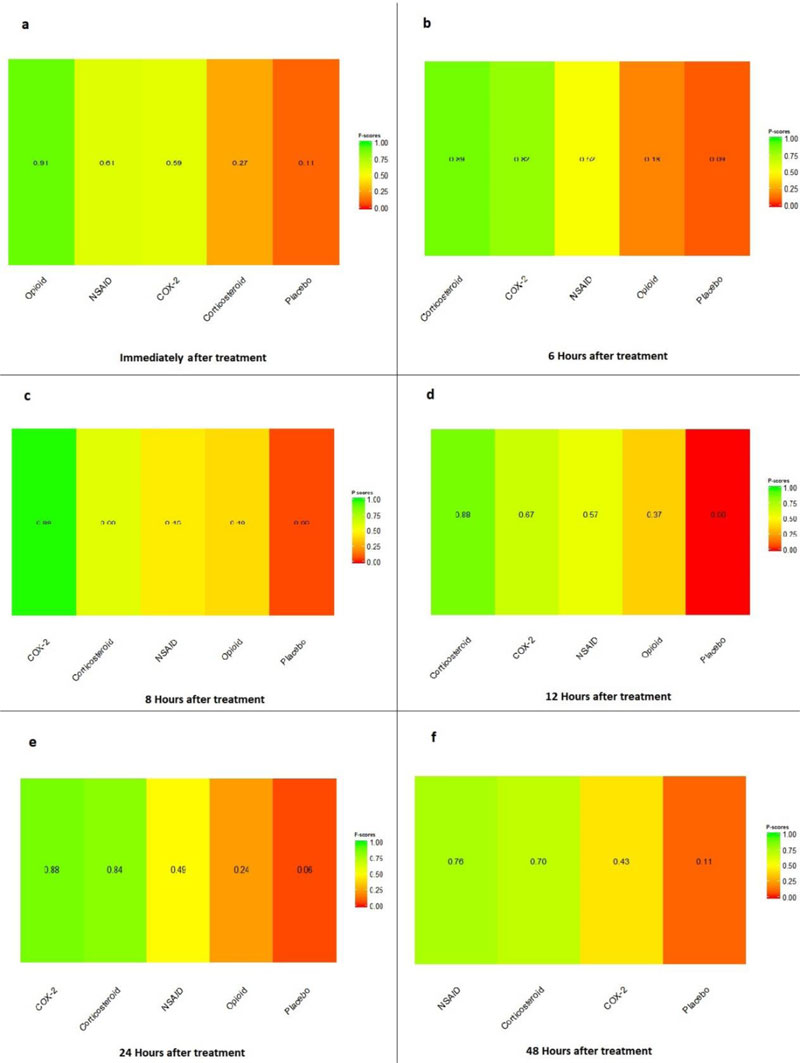
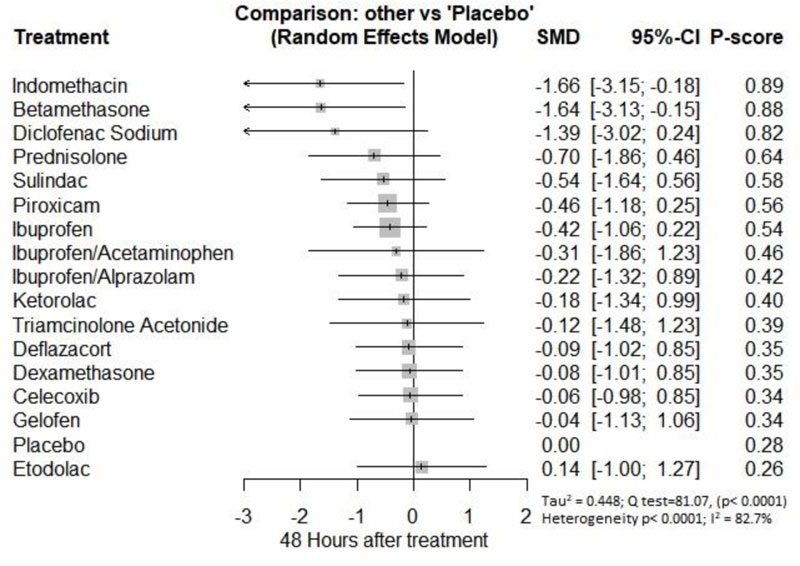
Twenty-four Hours after Procedure: Among all medications, COX-2 inhibitors were ranked as the best treatment for the reduction of postoperative pain when compared to placebo [SMD=-1.27, 95% CI (-2.10: -0.43), P-score=0.88]. Corticosteroids and NSAIDs also showed a significant reduction in pain score (SMD= -1.13 and SMD= -0.65, respectively). Pooled analysis was heterogeneous (Q=81.07; I2=82.7%; P<0.0001) due to the significant variation among the analyzed categories (Fig. 2e). Publication bias analysis showed a detected bias according to the Egger test (p=0.0008). Split analysis demonstrated that there was no significant difference between NSAIDs vs. Opioids and COX-2 inhibitors (Appendix Fig. 5). Network ranking graph showed the rank of categories at 24 hours after the procedure (Fig. 3e). League table is presented in Appendix Table 5.
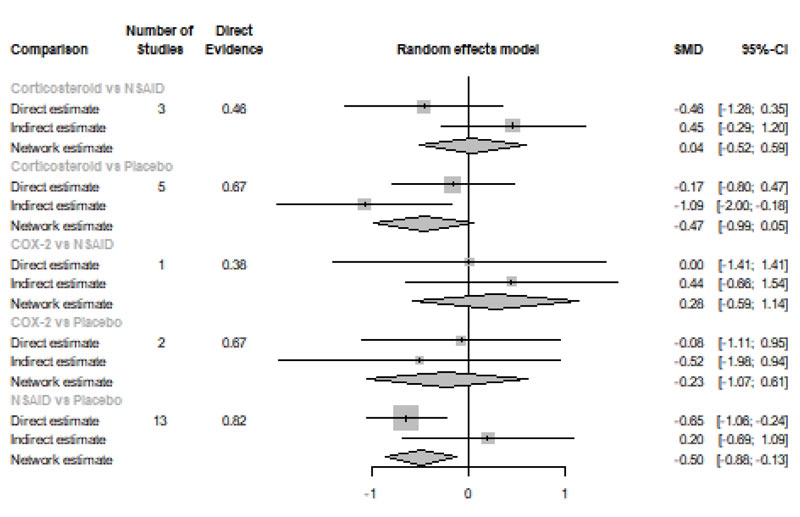
Forty-eight Hours after Procedure: Among all medications, only NSAIDs demonstrated a significant reduction in postoperative pain when compared to placebo [SMD=-0.50, 95% CI (-0.88: -0.13), P-score=0.76]. Pooled analysis was heterogeneous (Q=129.7; I2=83.8%; P<0.0001) due to the significant variation among the analyzed categories (Fig. 2f). Publication bias analysis showed that there was no detected bias according to the Egger test (p=0.16). Split analysis demonstrated that there was no significant difference among NSAIDs, Corticosteroids or COX-2 inhibitors (Appendix Fig. 6). Network ranking graph displayed the rank of categories at 24 hours after the procedure (Fig. 3f). League table is presented in Appendix Table 6.

3.3.2. Postoperative Pain for Treatment Intervention Categorized by Chemical Name
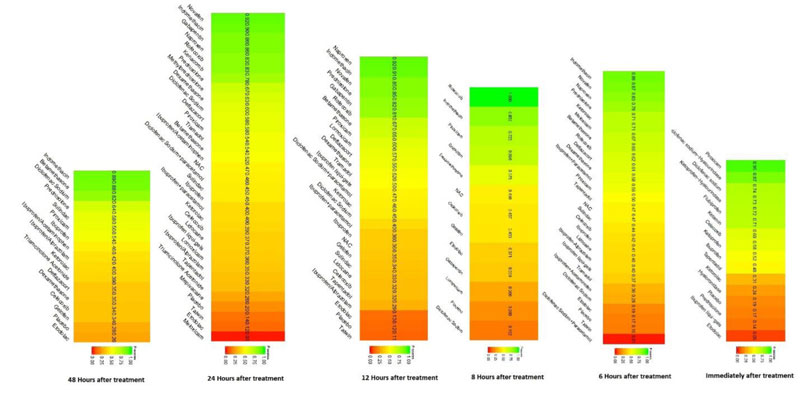
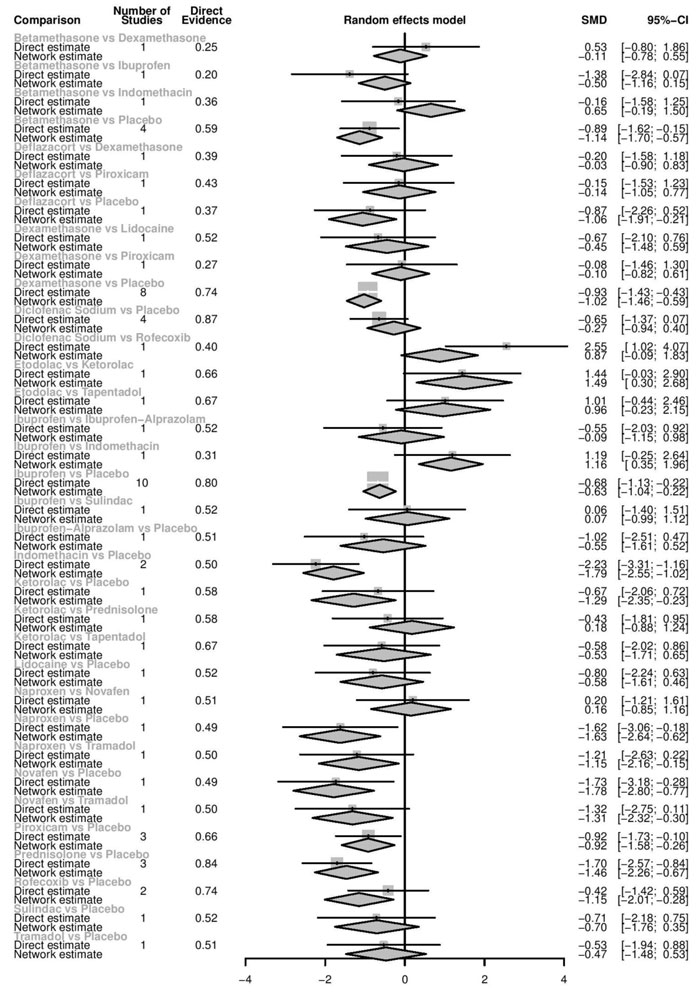
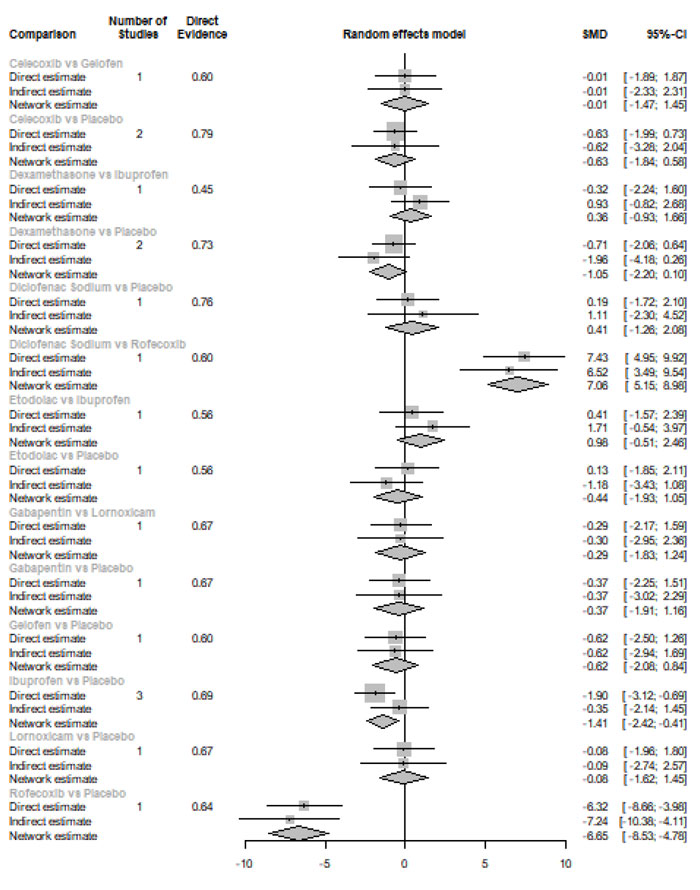
Network diagrams of all the eligible comparisons for primary outcomes according to the chemical name are presented in Fig. (4a-f).
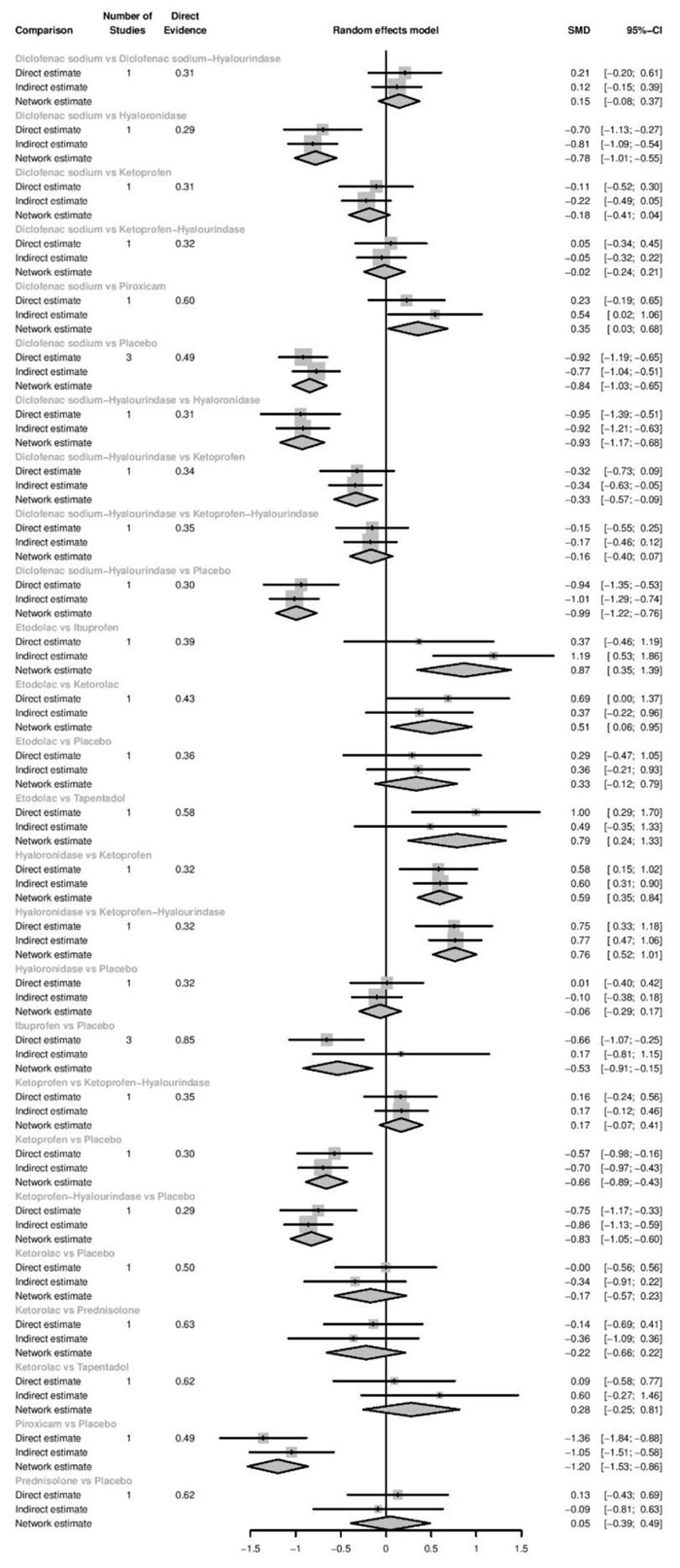
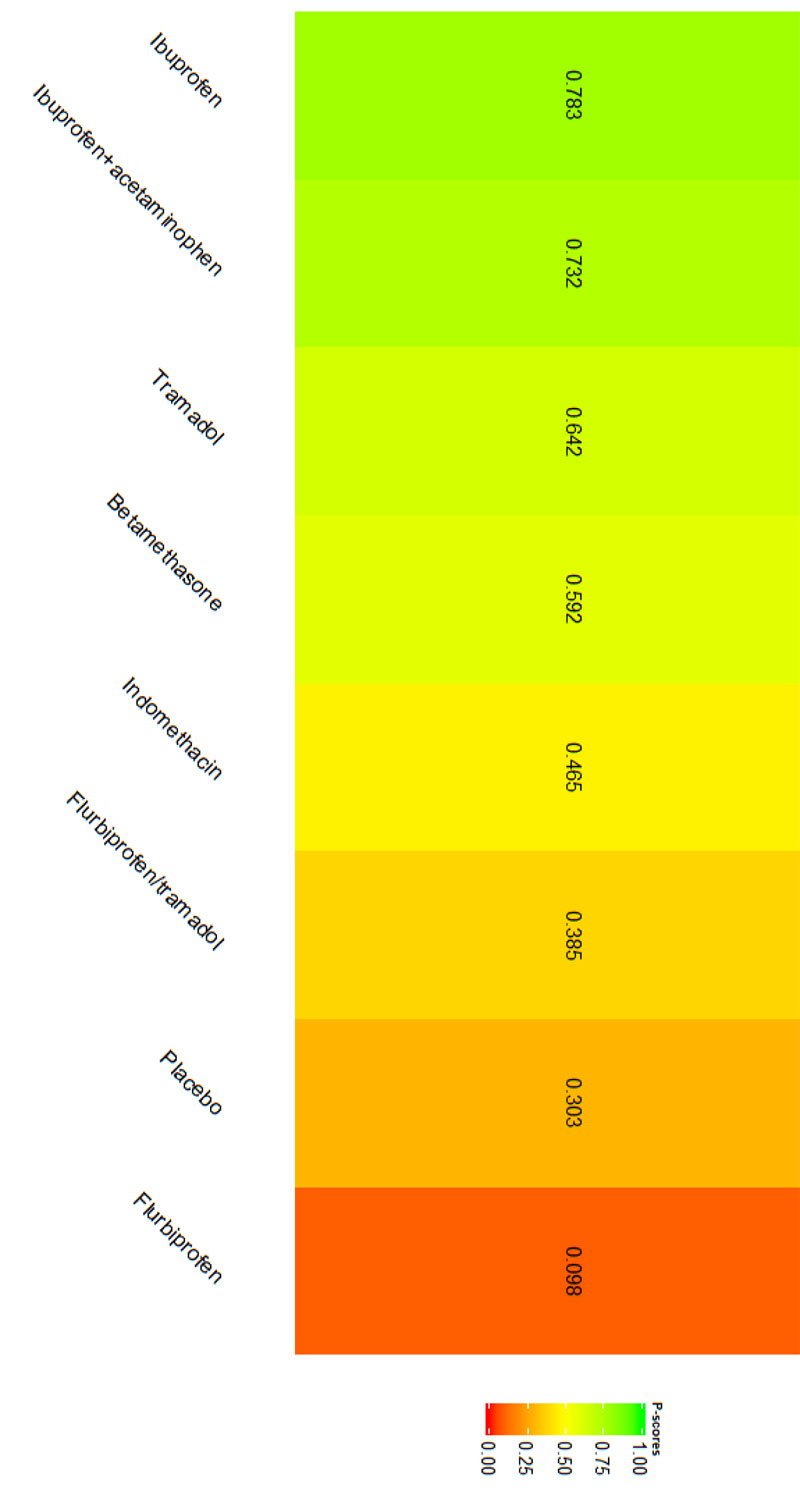
Immediately after procedure: Among all medications, Piroxicam was ranked as the best treatment for the reduction of postoperative pain [SMD= -1.20, 95% CI (-1.53: -0.86), P-score= 0.95]. Moreover, Diclofenac sodium, Flubiprofen, Ketamin, Ketoprofen, and Ibuprofen showed a significant reduction in pain after endodontic treatment. Pooled analysis was found to be homogenous (Q=23.89; I2=20.5%; P<0.97) (Appendix Fig. 7). Publication bias analysis showed that there was no detected bias according to the Egger test (p=0.66). The split analysis is presented in Appendix Fig. (8). Network ranking graph showed the rank of drugs immediately after the procedure (Fig. 5a).
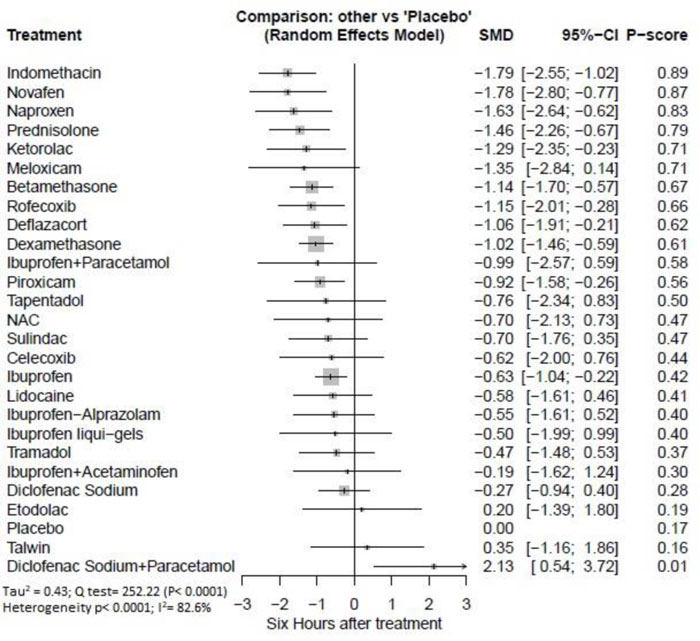
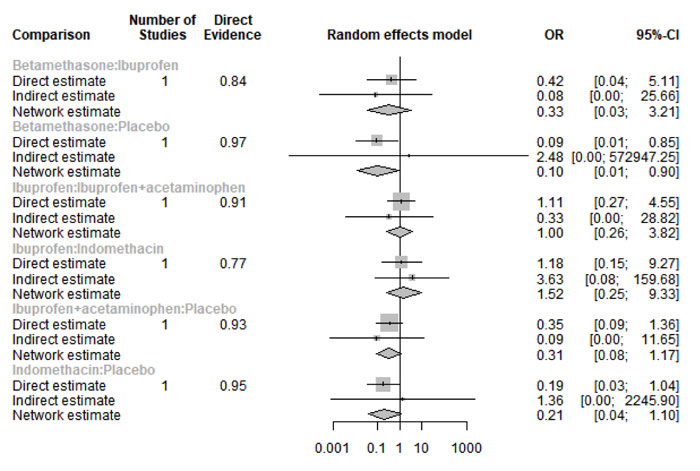
Six hours after procedure: Indomethacin was ranked as the best treatment for the reduction of postoperative pain [SMD= -1.79, 95% CI (-2.55: -1.02), P-score= 0.89]. Furthermore, Novafen, Naproxen, Prednisolone, Ketorolac, Betamethasone, Dexamethasone, Rofecoxib, Piroxicam, and Ibuprofen showed a significant reduction in pain after 6 hours of endodontic treatment. Pooled analysis was heterogeneous (Q=252.22; I2=82.6%; P<0.0001) due to the significant variation among the analyzed drugs (Appendix Fig. 9). Publication bias analysis showed a detected bias according to the Egger test (p<0.0001). The split analysis is presented in Appendix Fig. (10). Network ranking graph showed the rank of drugs 6 hours after the procedure (Fig. 5b).
Eight hours after procedure: At this period, only four drugs significantly reduced post-endodontic pain; Rofecoxib [SMD= -6.65, 95% CI (-8.53: -4.78), P-score= 1.00], Indomethacin [SMD= -2.39, 95% CI (-4.36: -0.42), P-score= 0.83], Piroxicam [SMD= -1.61, 95% CI (-2.97: -0.25), P-score= 0.72], and Ibuprofen [SMD= -1.41, 95% CI (-2.42: -0.41), P-score= 0.70]. Pooled analysis was heterogeneous (Q=82.04; I2=87.8%; P<0.0001) due to the significant variation among the analyzed drugs (Appendix Fig. 11). Publication bias analysis showed that there was no detected bias according to Egger test (p<0.15). The split analysis is presented in Appendix Fig. (12). Network ranking graph showed the rank of drugs 8 hours after the procedure (Fig. 5c).
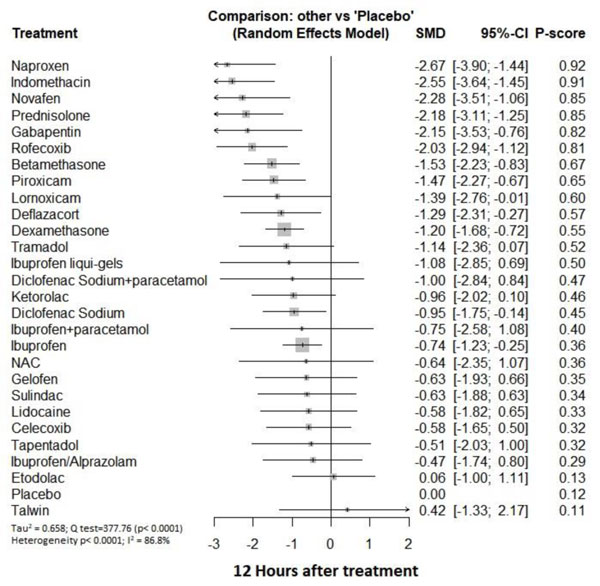
Twelve hours after procedure: Naproxen was ranked as the best treatment for the reduction of postoperative pain [SMD= -2.67, 95% CI (-3.90: -1.44), P-score= 0.92]. Furthermore, Novafen, Indomethacin, Prednisolone, Gabapentin, Betamethasone, Dexamethasone, Rofecoxib, Piroxicam, and Ibuprofen showed a significant reduction in pain after 12 hours of endodontic treatment. Pooled analysis was found to be heterogeneous (Q=377.76; I2=86.8%; P<0.0001) due to the significant variation among the analyzed drugs (Appendix Fig. 13). Publication bias analysis showed that there was a detected bias according to the Egger test (p<0.0001). The split analysis is presented in Appendix Fig. (14). Network ranking graph showed the rank of drugs 12 hours after the procedure (Fig. 5d).
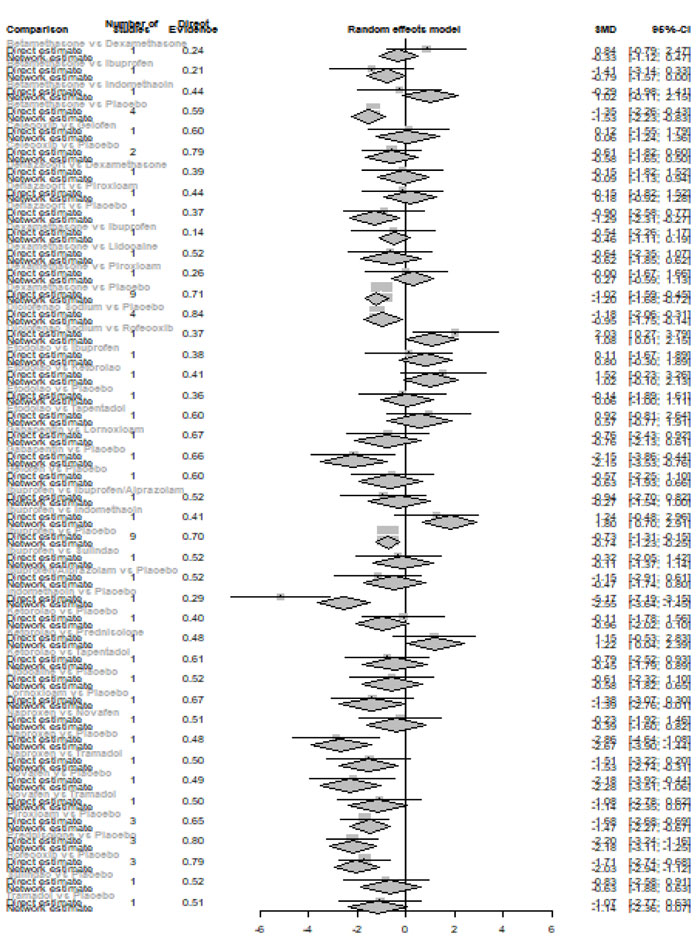
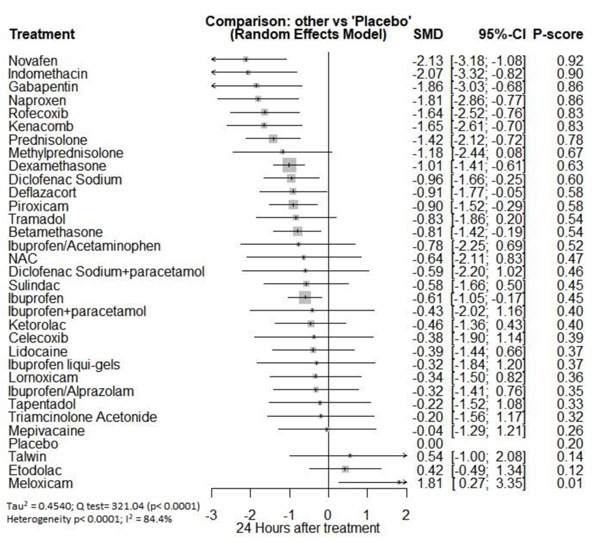
Twenty-four hours after procedure: Novafen was ranked as the best treatment for the reduction of postoperative pain [SMD= -2.13, 95% CI (-3.18: -1.08), P-score= 0.92]. Furthermore, Naproxen, Indomethacin, Prednisolone, Gabapentin, Diclofenac sodium, Betamethasone, Dexamethasone, Rofecoxib, Kenacomb, Piroxicam, and Ibuprofen showed a significant reduction in pain after 24 hours of endodontic treatment. Pooled analysis was observed to be heterogeneous (Q=321; I2=84.4%; P<0.0001) due to the significant variation among the analyzed drugs (Appendix Fig. 15). Publication bias analysis showed that there was a detected bias according to the Egger test (p=0.003). The split analysis is presented in Appendix Fig. (16). Network ranking graph showed the rank of drugs 24 hours after the procedure (Fig. 5e).
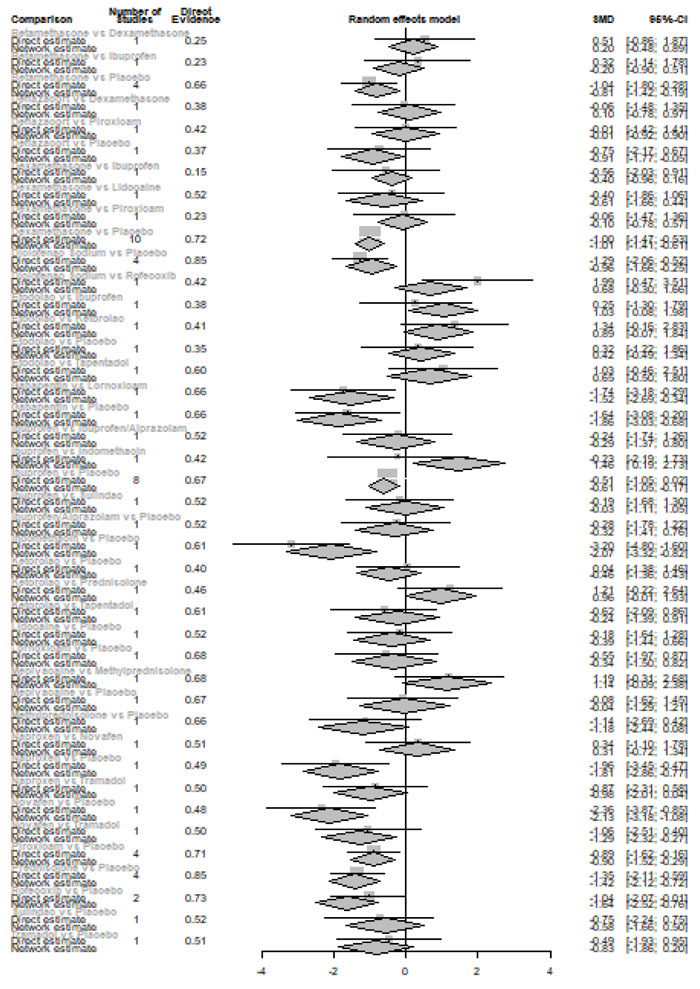
Forty-eight hours after procedure: Only indomethacin and betamethasone showed a significant reduction in postoperative pain [SMD= -1.66, 95% CI (-3.15: -0.18), P-score= 0.89] and [SMD= -1.64, 95% CI (-3.13: -0.15), P-score= 0.88], respectively. Pooled analysis was heterogeneous (Q=81; I2=82.7%; P<0.0001) due to the significant variation among the analyzed drugs (Appendix Fig. 17). Publication bias analysis showed that there was no detected bias according to the Egger test (p=0.32). The split analysis is presented in Appendix Fig. (18). Network ranking graph showed the rank of drugs 48 hours after the procedure (Fig. 5f).
3.4. Secondary Outcome: Adverse Events
3.4.1. Nausea
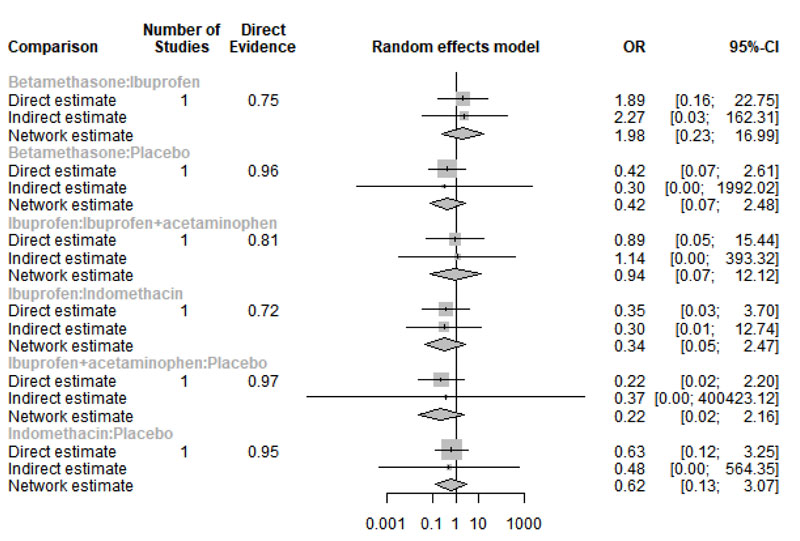
Our analysis showed that only five studies reported data regarding nausea [21-25]. Network graph included the following drugs: Indomethacin, ibuprofen, tramadol, betamethasone, flurbiprofen, and placebo (Appendix Fig. 19). Interestingly, among the tested drugs, no drug showed a significant increase in the risk/incidence of nausea, as shown in Appendix Fig. (20). Moreover, the ranking analysis demonstrated ibuprofen as the lowest drug associated with risk/incidence of nausea (Appendix Fig. 21). The split analysis is presented in Appendix Fig. (22).
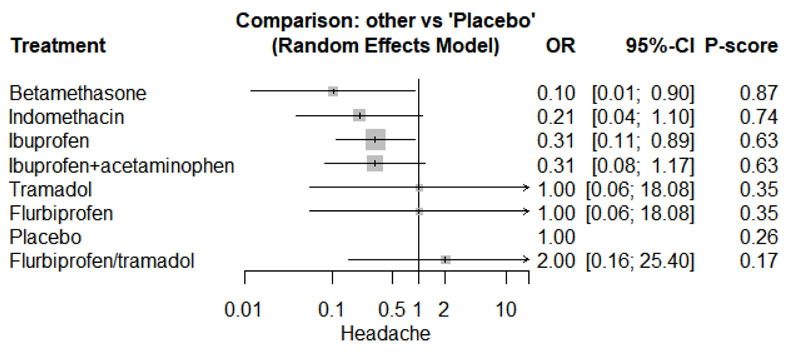
3.4.2. Headache
Only four studies reported data regarding headache [21-24]. Network graph included the following drugs: Indomethacin, ibuprofen, tramadol, betamethasone, flurbiprofen, and placebo (Appendix Fig. 23). Betamethasone and Ibuprofen showed a significant reduction in the risk/incidence of headache [OR= 0.10, 95% CI (0.01: 0.90), P-score= 0.87] and [OR= 0.31, 95% CI (0.11: 0.89), P-score= 0.63], respectively (Appendix Fig. 24). Moreover, the ranking analysis demonstrated that betamethasone was the lowest drug associated with risk/incidence of headache (Appendix Fig. 25). The split analysis is presented in Appendix Fig. 26.
3.4.3. Other Adverse Events
Salapoor et al. [24] reported one case and Menhinick et al. [21]reported three cases of sweating due to using ibuprofen. Regarding dizziness, Shantiaee et al. [24] reported three cases with dexamethasone, and Sethi et al. [23]reported four cases with Tapentadol and Etodolac. In terms of vomiting and heartburn, three cases were recorded for each Tapentadol and Etodolac [23].
4. DISCUSSION
To the best of our knowledge, this is the largest and most updated systematic review and network meta-analysis that was conducted to evaluate the current evidence regarding the effect of pre- and postmedication for reducing the postendodontic pain. In this study, we included a total of 62 RCTs in the systematic review. Out of them, 50 studies were included in the network meta-analysis (NMA). NMA was conducted on the basis of pharmacological or chemical name groupings in order to identify the effect of classification of the medications given pre- or postendodontic care on postoperative pain during the following periods: immediately, 6, 8, 12, 24, 48 hours after the procedure. Opioids were ranked first in the pharmacologic group for reducing pain immediately after the procedure. Moreover, it showed a significant reduction at 12 hours after the procedure. Corticosteroids were ranked first as the best treatment for the reduction of postoperative pain at 6 and 12 hours with a significant reduction in postoperative pain scores [SMD= -1.18, 95% CI (-1.51: -0.85)] and [SMD= -1.39, 95% CI (-1.77: -1.02)], respectively. COX-2 were ranked as the best treatment for the reduction of postoperative pain at 8 and 24 hours with a significant reduction in postoperative pain scores [SMD= -2.86, 95% CI (-6.05: -1.66)] and [SMD= -1.27, 95% CI (-2.10: -0.43)], respectively. NSAIDs significantly reduced the postoperative pain scores in all durations. Based on the chemical name, piroxicam was superior immediately after the procedure, whereas indomethacin followed by novafen, naproxen, and prednisolone was found to be effective at 6 hours. At 12 and 24 hours, naproxen and Novafen followed by indomethacin were ranked first. However, at 48 hours, only indomethacin and betamethasone were effective. The safety profile of test drugs was acceptable except for some events of nausea, vomiting, and headache.
Clinically, it has been reported that patients with periapical diagnosis of an Acute Apical Periodontitis (APP) or Phoenix Abscess are more likely to require additional medication to relieve post-endodontic pain compared to a periapical diagnosis of a Normal Periapex, a Chronic Apical Periodontitis (CAP), or a Chronic Apical Abscess (CAA) [26, 27]. Therefore, it seems rational to minimize occlusion after root canal therapy on the tooth, which is harmful to percussion. Occlusal reduction in patients with teeth that initially show pulp vitality, percussion sensitivity, preoperative pain and/or absence of periradicular radiolucency has been recommended to prevent postoperative pain [28]. On the other hand, CAA or CAP consists of a radiolucency at the root apex, a draining fistula (sinus tract), and usually no pain in percussion.
Nagendrababu et al. [17] conducted NMA for the same purpose; however, they only included 16 RCTs and reported results for only three durations. In terms of adverse events, they reported a descriptive result and did not conduct a pooled analysis. In conclusion, they stated that the use of piroxicam or prednisolone would be the premedication of choice. We agree that these drugs are promising and show a significant effect; however, we believe that indomethacin, Novafen, naproxen, betamethasone have a better effect and longer duration.
In the NMA of Shirvani and colleagues, they aimed to investigate the efficacy of NSAIDs and paracetamol in reducing postendodontic pain. They did not include corticosteroids or opioids; therefore, they enrolled only 27 articles. They analyzed the data at four durations immediately, 6, 12, and 24 hours after the procedure. They performed a meta-regression which demonstrated that combination therapy did not reduce the pain significantly (OR= -0.88, 95% CI (-2.05, 0.28), p= 0.1). Moreover, they showed that the systemic administration was more efficient than oral administration (OR= -1.17, 95% CI (-1.93, -0.41), p= 0.004) and (OR= 4.24, 95% CI (2.62, 5.86), p<0.001), respectively. Finally, they recommended the use of multiple-dose regimens of NSAIDs during the postoperative period to achieve most efficacy (29). Smith et al. (30) found that the elimination of 6 hours of postendodontic pain with ibuprofen 600 mg and ibuprofen 600 mg + acetaminophen 1000 mg was more effective than placebo. They analyzed studies that evaluated the efficacy of pre- and postmedication for endodontic treatment on pain. They showed that ketoprofen 50 mg and naproxen 500 mg might be more effective than ibuprofen 600 mg at 6 hours postoperative.
5. Limitations
This study possessed some limitations: 1) Heterogeneity was observed in all analyses, which can be explained by the extensive variation in types of drugs, dosage, mechanism of action, and mode of administration. Moreover, the different types of teeth of participants with varied demographics may influence the applicability of our findings. However, all studies were conducted in hospitals, universities or clinics where the numbers and experience of operators were diversified, which could further encourage our findings to be generalized. 2) We could not conduct a subgroup analysis according to the regimen doses because of insufficient data.
CONCLUSION
In conclusion, the current evidence suggests that pre- and postmedication have the ability to reduce postoperative pain after nonsurgical root canal treatment. Corticosteroids and COX-2 inhibitors showed significant control of the pain up to 12 hours after administration. However, NSAIDs demonstrated a high efficacy from administration and until two days after treatment. Indomethacin, Novafen, prednisolone, and Naproxen were ranked as first in most analyzed durations. The use of narcotic agents before and post-nonsurgical root canal procedures for postoperative pain control and improving the quality of life needs further research.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Hussien Ahmed of MRSclin for the editorial and statistical support.
APPENDIX
Primary outcome: Postoperative Pain Treatment Intervention Categorized by Pharmacologic Group








| Corticosteroid | - | - | - | - |
|---|---|---|---|---|
| 0.47 (-0.86; 1.80) | COX-2 | - | - | - |
| 0.45 (-0.26; 1.15) | -0.02 (-1.18; 1.13) | NSAID | - | - |
| 0.98 (-0.05; 2.01) | 0.51 (-0.87; 1.89) | 0.53 (-0.22; 1.29) | Opioid | - |
| -0.18 (-0.88; 0.53) | -0.65 (-1.78; 0.48) | -0.63 (-0.89; -0.36) | -1.16 (-1.96; -0.36) | Placebo |
| Corticosteroid | - | - | - | - |
|---|---|---|---|---|
| -0.07 (-0.90; 0.75) | COX-2 | - | - | - |
| -0.51 (-0.89; -0.13) | -0.43 (-1.22; 0.35) | NSAID | - | - |
| -1.05 (-1.76; -0.34) | -0.98 (-1.96; 0.01) | -0.54 (-1.17; 0.09) | Opioid | - |
| -1.18 (-1.51; -0.85) | -1.10 (-1.86; -0.34) | -0.67 (-0.93; -0.41) | -0.13 (-0.77; 0.52) | Placebo |
| Corticosteroid | - | - | - | - |
|---|---|---|---|---|
| 1.63 (-0.07; 3.33) | COX-2 | - | - | - |
| -0.40 (-1.75; 0.95) | -2.03 (-3.27; -0.79) | NSAID | - | - |
| -0.48 (-2.62; 1.65) | -2.11 (-4.20; -0.03) | -0.08 (-1.85; 1.68) | Opioid | - |
| -1.23 (-2.48; 0.02) | -2.86 (-4.05; -1.66) | -0.83 (-1.54; -0.11) | -0.75 (-2.51; 1.02) | Placebo |
| Corticosteroid | - | - | - | - |
|---|---|---|---|---|
| -0.19 (-1.00; 0.62) | COX-2 | - | - | - |
| -0.29 (-0.72; 0.13) | -0.10 (-0.86; 0.66) | NSAID | - | - |
| -0.56 (-1.28; 0.16) | -0.37 (-1.32; 0.59) | -0.27 (-0.89; 0.36) | Opioid | - |
| -1.39 (-1.77; -1.02) | -1.20 (-1.92; -0.48) | -1.10 (-1.39; -0.81) | -0.84 (-1.48; -0.20) | Placebo |
| Corticosteroid | - | - | - | - |
|---|---|---|---|---|
| 0.13 (-0.75; 1.01) | COX-2 | - | - | - |
| -0.48 (-0.85; -0.11) | -0.61 (-1.47; 0.25) | NSAID | - | - |
| -0.87 (-1.59; -0.15) | -1.01 (-2.08; 0.07) | -0.39 (-1.08; 0.29) | Opioid | - |
| -1.13 (-1.44; -0.83) | -1.27 (-2.10; -0.43) | -0.65 (-0.94; -0.37) | -0.26 (-0.95; 0.43) | Placebo |
| Corticosteroid | - | ||
|---|---|---|---|
| -0.24 (-1.21; 0.73) | COX-2 | ||
| 0.04 (-0.52; 0.59) | 0.28 (-0.59; 1.14) | NSAID | |
| -0.47 (-0.99; 0.05) | -0.23 (-1.07; 0.61) | -0.50 (-0.88; -0.13) | Placebo |


