All published articles of this journal are available on ScienceDirect.
Potential Antibacterial Flavonoid from Buah Merah (Pandanus conodieus Lam.) Against Pathogenic Oral Bacteria of Enterococcus faecalis ATCC 29212
Abstract
Background:
Dental caries is an oral disease generated by pathogenic bacteria, Enterococcus faecalis, which is most frequently found in teeth with pulp necrosis. On the other hand, the use of the medicinal plant to treat pathogenic disease, including caries is an alternative option, which consumes synthetic drug having a side effect.
Objective:
The purpose of this study is to isolate antibacterial agents from Buah Merah (Pandanus conoideus Lam) and to test the antibacterial activity of those compounds against Enterococcus faecalis ATCC 29212.
Methods:
Isolation of the antibacterial constituents from Buah Merah used a combinational column chromatography technique which include a normal and reversed-phase. The chosen fraction of each separation is based on the most active fraction. The compounds at various concentrations, 1000 - 20000 μg/mL, were assessed against E. faecalis ATCC 29212 by agar disc diffusion method, and chlorhexidine 2000 μg/mL was used as a positive control.
Results:
Four compounds isolated from Buah Merah were determined as flavonoid 1, diterpenoid 2, and two fatty acid derivatives 3 and 4. The compounds were then tested against E. faecalis cultured to find inhibition zones, and the study found that only compound 1 identified as Quercetin-3-O-glucose showed an inhibited zone 88 mm at 20000 ppm.
Conclusion:
This study demonstrated that ethyl acetate fraction of Buah Merah contains an antibacterial flavonoid active against E. faecalis. This research gives information for the use of this plant in herbal medicine and contributes to the necessity of a new antibacterial agent for oral infectious disease. Moreover, this data can be based on information to find the substituted antiseptic applied in the dentistry field.
1. INTRODUCTION
Dental caries, one of the infectious oral disease, is caused by cariogenic bacteria. Many approaches have been tracked to prevent them, such as inhibition of the bacteria growth, reduction of bacterial plaque formation, increasing dental care, and healthy diet modification [1]. In endodontic treatments, one of the most prevalent causes of treatment failure are microorganisms in root canals. Among the numerous bacteria, Enterococcus faecalis is one of the most frequently found in teeth with pulp necrosis [2].
In dentistry, phytomedicine has been used as anti-inflammatory, antibiotic, analgesic, sedative agents and also as endodontic irrigants. Studies on phytotherapeutic agents against multi-resistant bacteria may contribute to the development of effective new drugs in the treatment of severe infections [3]. By the resistance issue of the antibacterial agent, various efforts have been made to the discovery of antibacterial substances and effective mechanisms that could reduce the causative agents of dental diseases. One of the antibacterial agent sources is natural products that have been used by society for some purposes in a long time. Many herb plants that are used to cure some diseases contain bioactive compounds. One of the medicinal plants is Pandanus conoideus Lam. that has a local name Buah Merah, in Indonesia.
As the search continues for new antibacterial compounds against pathogenic oral bacteria from Indonesian plants [4-6], Buah Merah (P. conoideus Lam.), an edible medicinal plant available easily in Papua Island, Indonesia belongs to genus Pandanus which has been used as a traditional medicine to treat some degenerative disease. Some studies demonstrated that fruits extracts are cytotoxic, anti-diabetic, anticancer, antibacterial, and antioxidant [7, 8]. A previous study stated that ethyl acetate extract of Buah Merah can inhibit the growth of Candida albicans with MIC of 1.5% [9]. This extract is the most active extract against Enterococcus faecalis, Sterprococcus mutans, and Streptococcus sanguinis [10]. Some chemical constituents of Buah Merah include α- and β-carotenoids, fatty acids, α- and β-cryptoxanthin, tocopherol, steroids [11-13]. As a potential source of antibacterial agent, Buah Merah extract seems to act against pathogenic bacteria, while the active constituents have not been discovered yet. This study describes the identification and antibacterial assessment against pathogenic oral bacteria of flavonoids compounds from the fruits of Buah Merah.
2. MATERIALS AND METHODS
2.1. Plant Materials and Chemicals
Fresh Buah Merah (P. Conodieus Lam.) was collected from Papua, Indonesia, in June 2017. The sample was identified at the Laboratory of Plants Taxonomy - Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Bandung, Indonesia. Materials for the purification of the compounds used Kiesel G 60 silica gel resins (No. Cat 1.07734.1000, Merck) and the ODS of LiChroprep RP-18 (Merck, Darmstadt, Germany). Meanwhile, the Kiesel gel 60 F254 and RP-18 F254S (Merck) are used for Thin-Layer Chromatography analysis. Then, deuterated solvents were purchased from Merck Co. Ltd. and Sigma Aldrich Co. Ltd. (St. Louis, MO, USA).
2.2. Test Organisms
The Enterococcus faecalis with the ATCC 29212 type was used in the antibacterial test. The media used for the bacteria growth are Muller Hinton broth and agar media (CM0405 and CM0337, Oxoid). The antibiotic assay disc for the susceptibility testing was purchased form sigma Aldrich (Whatman, diam 6 mm). Meanwhile, Fosfomycin and chlorhexidine, as the positive control, were purchased from Merck Co. Ltd. and Sigma Aldrich.
2.3. Instruments
For the characterization of chemical structure, this study uses a 500 MHz FT-NMR spectrometer (Varian ECA 500 JOEL, Japan) to record the NMR spectra, a Perkin Elmer Spectrum One FT-IR spectrometer (Buckinghamshire, England) to determine IR spectra, ES-MS Spectrometer (UPLC MS/MS TQD type, Waters) to get Mass Spectra data, and a UV spectrophotometer (Shimizu, Japan) to provide UV spectra. Meanwhile, the instrument for the antibacterial testing was measured on Biochrom EZ Read 400 Elisa Reader. Other instruments that support the test are incubator Memmert, autoclave machine HVE-50 Hirayama, laminar airflow, and anaerobic jar (for anaerobic condition, Oxoid).
2.4. Preparation of Extract
The collected fresh fruits were washed and cut into small sizes. The samples were extracted with methanol and then partitioned with water-n-hexane and water-ethyl acetate, respectively. The extract obtained was filtered, concentrated in vacuo, and made into a series of concentrations for phytochemical screening and antibacterial activity test.
2.5. Preliminary Phytochemical Screening
All Buah Merah’s extract (Methanol, n-hexane, ethyl acetate, and water extracts) tested in presence or absence of the various secondary metabolite constituents were carried out using standard methods [14, 15].
2.6. Isolation Active Compounds 1-4
The antibacterial compounds from Buah Merah (P. Conodieus Lam.) were separated and purified by bioactivity-guided in isolation steps. The Buah Merah fruit was extracted with methanol and then subsequently partitioned between n-hexane and ethyl acetate, respectively. The active extracts were separated and purified by combinations of column chromatography methods on Silica G 60 (normal phase) and ODS RP-18 (reverse-phase). The purity of isolated compounds was evaluated by TLC analysis on Silica G 60 F254 and ODS RP-18 F254S plates, and the compounds on plates were visualized under UV light (254 or 356 nm) and spraying with 10% H2SO4 in EtOH followed by heating. The purity of active compounds was analyzed by 1D and 2D TLC analyses, respectively.
2.7. Structure Determination of Compounds 1-4
The structure determination of active compounds was conducted by detail comprehensive analysis data of spectroscopic methods including Ultra-violet (UV) spectrum, Infra-red (IR) spectrum, 1D and 2D-NMR spectra (1H-NMR, 13C-NMR, DEPT 135°, HMQC, 1H-1H COSY, HMBC) and mass spectrometry (MS) spectrum.
2.8. Antibacterial Activity of Extract and Compounds 1-4
The antibacterial effects evaluations of the extracts and compounds 1-4 resist E. faecalis ATCC 29212 was conducted using the Kirby-Bauer (disc diffusion) method [16]. This assay was used to measure how E. faecalis sensitivity or resistance to compounds. Each compound was diluted with a methanol-water mixture (1:1, v/v) and chlorhexidine as a positive control was diluted in water. All compounds and control were assayed with a concentration of 1000, 2500, 3000, 5000, 10000, and 20000 μg/mL. Then, each sample (20 μL) was impregnated on paper discs (6 mm). Furthermore, the discs that contained the sample were placed on the surface of the agar media. After incubation for 48 h, the inhibit zone of the sample was measured.
Furthermore, the antibacterial assay of MIC and MBC against E. faecalis ATCC 29212 for compounds 1 and 2 were assessed by dilution method in micro-scale use 96-well microplate [16, 17]. The first step of this test is to grow the bacteria in the sterile broth medium incubated at 37°C for 48 h under anaerobic conditions. Then, the medium and sample were loaded in the microplate wells following the test layout and diluted by diluting serial two-folds. Next, 0.5 bacteria McFarland were added to the well and incubated at 37°C for 24 h. The analysis of the MIC valued was determined by the bacterial turbidity values shown by the microplate reader at 620 nm. Finally, the solution in the wells was spread on the agar medium to observe whether bacteria are growing or not, so that the MBC value can be determined. Chlorhexidine and fosfomycin, the positive controls, were diluted in water and methanol. Meanwhile, the whole sample was dissolved in a solvent suitable with the characteristic of the sample (methanol or water).
3. RESULTS
3.1. Preparation of Buah Merah Extract
The fresh fruits of Buah Merah (10 Kg) were cut into small pieces and extracted with MeOH for 3x24 hours, and the extract solution was evaporated with a rotary evaporator in vacuo at 40°C. The concentrated extract provided after the evaporating process was 106.60 g.It was then partitioned between n-hexane-H2O (2:1), and followed by EtOAc-H2O yielded crude extract of n-hexane (45.08g), EtOAc (8.61g) and H2O (9.85g), respectively.
3.2. Preliminary Phytochemical Screening
The chemicals constituents' identity of herbals plant is one of the important aspects as a guide to determine chemical structure and selection of separations methods. To identify the secondary metabolite contents, small cutting of fresh Buah Merah was extracted using various solvents with different polarities of n-hexane, ethyl acetate, methanol, and water, respectively. All of the extracts were subjected to phytochemicals analysis to identify their secondary metabolites groups. As shown in Table 1, the results indicated that all extracts contain flavonoids, terpenoids, and alkaloids, except for the n-hexane extract. It can be suggested that Buah Merah extract contained important secondary metabolites as new sources of bioactive as antibacterial compounds.
3.3. Antibacterial Activity of Extracts
As the main target of this research for discovering new antibacterial agents, all extracts of Buah Merah were evaluated for their antibacterial activity. The extracts were subjected to antibacterial assay against E. faecalis, and their inhibition zone values are presented in Table 2. The data presented in Table 2 showed that the ethyl acetate fraction has the biggest inhibition zone values that are 7.5, 8.6, and 9.3 mm at 10000, 20000, and 40000 ppm, respectively, so that, it is the most active fraction. This data is very interesting and important as a guide for further isolation, separations, and purification steps of antibacterial constituents, because in some published papers, ethyl acetate extract was reported to contain bioactive compounds as antibacterial with unique chemical structures.
3.4. Isolation Procedure of Compounds 1-4
Based on the preliminary antibacterial assay of Buah Merah extracts against E. faecalis, the most active of ethyl acetate (8.61g) was furthermore separated on silica G 60 (300 g, 70-230 mesh, Merck, Munich, Germany) eluted with a stepwise of 10% n-hexane-EtOAc, to give twenty-one fractions. The fraction XI (0.73 g) was subjected to an RP-C18 column, eluted with a gradient 1% of H2O-MeOH to yields 1 (20.50 mg) and 2 (16.60 mg), while fraction IV (1.06 g) was subjected to an RP-C18 column, eluted with a stepwise of 1% H2O-MeOH to yield 3 (12.60 mg) and 4 (15.50 mg). The Rf values for compounds 1 and 2 were 0.73 and 0.63 on TLC (RP-18 F254, MeOH-H2O 7:3) and while compounds 3 and 4 were 0.54 and 0.30 on TLC (RP-18 F254, MeOH 100%), respectively.
3.5. Structure Determination of Compounds 1-4
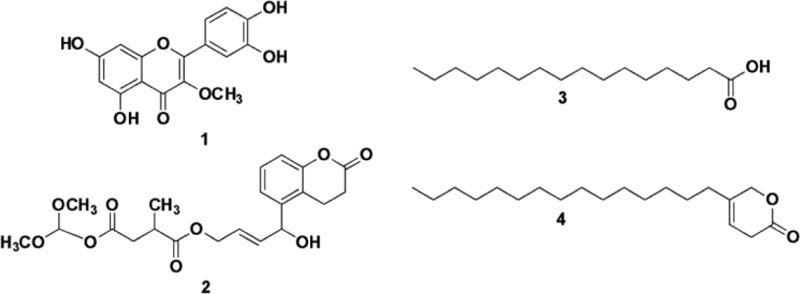
The structural determination of the antibacterial compounds was conducted by comprehensive measurement and analysis of their UV, IR MS and 1D and 2D NMR spectral data. Compound 1 was isolated as a yellow solid and the molecular weight (m/z) of 1 was 315.41 (C16H12O7) based on the mass spectra in mode negative ion. The IR spectra indicated the hydroxyl and carbonyl group at absorptions of 3233 and 1608 cm-1, respectively. The 13C-NMR spectrum showed that compound 1 had sixteen carbon signals including a carbon oxygenated at δC 60.1 ppm, a carbonyl at δC 180.0 ppm and fourteen carbons conjugated from aromatic at δC 94.8-166.0, while the 1H-NMR spectrum showed that 1 had twelve protons, three protons were methyl oxygenated (O-CH3) at δH 3.78 ppm (δC 60.6 ppm), five protons aromatic methines a t δH 6.19-7.62 ppm (δC 94.8-122.4 ppm), the sp2 protons of at δH 6.19 and 6.38 ppm (δC 99.8 and 94.8 ppm) with values J = 1.8 for H-6 and H-8 those represented two protons were interconnected for a meta-position, and sp2 protons at δH 6.90 and 7.52 ppm (δC 116.5 and 122.4 ppm)had J = 8.5 for H-5' and H-6' that correspond with the protons in an ortho position. Based on the interpretation of all spectra data and verified to previous literature, compound 1 was identified as flavonoids of Quercetin-3-methyl ether, as shown in Fig. (1) [18].
Compound 2 was isolated as a white solid, and its molecular weight (m/z) of 2 was 421.36 (C21H24O9), as shown on the [M + H]+ peak in mass spectra. The IR data indicated a hydroxyl and carbonyl group at the absorption of 3480 and 1650 cm-1, respectively. 13C-NMR Data of 2 represented twenty-one carbon signals corresponding to three carbonyls ester at δC 170.1; 175.6 and 177.8 ppm, a methyl carbon at δC 17.3 ppm, two oxygenated methyls at δC 56.4 ppm, methylene at δC 38.4 ppm, oxygenated methylene at δC 65.3 ppm, three quaternary carbons at δC 1527; 148.7 and 123.1, and ten methine carbons at δC 37.1, 52.4, 52.5, 112.7, 113.8 (x2), 115.9, 121.1 and 125.3 (x2) ppm. The 1H-NMR spectrum of 2 then showed twenty-four protons, including six protons for two oxygenated methyls (O-CH3) at δH 3.88 ppm (δC 56.4 ppm), three methyl protons at δH 1.19 ppm (δC 17.3 ppm), two methylene protons at δH 2.66; 2.44 ppm (δC 38.4 ppm), four protons for two oxygenated methyl and oxygenated at δH 3.68 ppm (δC 52.4, 52.5 and 65.3 ppm), two protons at δH of 6.83 ppm were protons of two olefinic carbons at δC 112.7 and 115.9 ppm with J = 8.45 values for H-11 and H-9, indicating that the two protons were interconnected and bound to neighboring carbon, four protons of two methylenes at δH 7.55 ppm (δC 113.8 and 125.3 ppm) and a methyl proton at δH 6.73 ppm (δC 121.1 ppm). From the spectral data analysis and comparison with published data, the structure of compound 2 suggests that it is a derivative of succinic acid, as shown in Fig. (1), and only the first time reported from this plant. Two other compounds were identified as a saturated fatty acid derivative of hexadecanoic acid or palmitic acid (3) and 5-pentadactyl-3H-Piran-2(6H)-on (4) as well as by comparison of their spectral data with a previously published study as shown by Fig. (1) [19]. Based on the data, the most active of ethyl acetate extract, four compounds were isolated and identified as flavonoid 1, diterpenoid 2, and two fatty acid derivatives 3 and 4. The structure of compounds 1-4 is shown in Fig. (1).
3.6. Antibacterial Activity of Compounds 1-4
The bioactivity evaluation isolated compounds then performed against one of the important pathogenic oral bacteria by in vitro assay. The activities of compounds 1-4 against E. faecalis can be observed to inhibit any zone of the tested compound when grown using the Kirby-Bauer method. As shown in Table 3, the susceptibility test of the samples was performed in six concentrations at 1000, 2500, 3000, 5000, 10000, and 20000 ppm, with chlorhexidine and fosfomycin, as positive control and MeOH as a negative control to show that it does not effect the growth of bacteria.
Compound 1 showed antibacterial activity with inhibition zones of 8.8 mm at 20000 ppm, while other compounds were inactive. Further susceptibility assay of 1 demonstrated that the MIC and MBC values were 2500 and 5000 ppm, respectively.
| No. | Fractions | Secondary Metabolites | ||
|---|---|---|---|---|
| Flavonoids | Terpenoids | Alkaloids | ||
| 1 | n-Hexane | - | + | + |
| 2 | EtOAc | + | + | + |
| 3 | MeOH | + | + | + |
| No. | Fractions | Inhibition zone (mm) and concentrations (ppm) | ||
|---|---|---|---|---|
| 10000 | 20000 | 40000 | ||
| 1 | n-Hexane | 0.00 | 0.00 | 0.00 |
| 2 | EtOAc | 7.50 | 8.60 | 9.30 |
| 3 | MeOH | 0.00 | 0.00 | 0.00 |
| 4 | Chlorhexidine | 0.00 | 16.02 | 0.00 |
| No. | Compounds | Inhibit Zone (mm) at Concentration (ppm) | |||||
|---|---|---|---|---|---|---|---|
| 1000 | 2500 | 3000 | 5000 | 10000 | 20000 | ||
| 1 | 1 | - | - | - | 0.00 | 0.00 | 8.80 |
| 2 | 2 | 0.00 | 0.00 | 0.00 | - | - | - |
| 3 | 3 | - | - | - | 0.00 | 0.00 | 0.00 |
| 4 | 4 | - | - | - | 0.00 | 0.00 | 0.00 |
| 5 | Chlorhexidine | - | 11.90 | - | - | - | - |
| 6 | Methanol | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
4. DISCUSSION
The discovery of new natural dental medicine from medicinal plants as antibacterial agents for dental caries is increasingly developing in the scientific effort to characterize the benefits, side effects, as well as to determine a safe dosage for application. Recently, the carries disease treatment is effected by infection pathogenic oral bacteria of E. faecalis, S. mutans, and S. sanguinis especially treated by 2% chlorhexidine as the gold standard [20, 21], with some side effects of discoloration of the teeth and drug resistance. Previous papers reported some alternatives to the prevention of dental plaque related diseases and the improvement of dental health by complement or substitute active antibacterial agents, i.e., probiotics, xylitol, and sea salt [22-28]. According to these references to solve this problem, there is a need to discover and develop new antibacterial compounds with good effects of more selective, effective, and efficient with no or a very limited negative side effect.
Natural products are potential sources that synthesize diverse bioactive compounds that act as antifungal and antibacterial agents for therapeutically viable new antibacterial agents. This study presents data on the antibacterial activity of edible plants, which was selected based on the fact that it was consumed as daily health food [29, 30].
Drugs derived from plants are the secondary metabolites that play an important role in defense of plants against things that threaten their survival, such as pathogenic bacteria, fungi, viruses, and environmental stress factors, although they are not part of the main metabolism of plant life. In Indonesia, Buah Merah (P. conoideus Lam.) has been used to treat infectious diseases for a long time. The present study isolates antibacterial flavonoids from Buah Merah and evaluates its antibacterial activity resist E. faecalis ATCC 29212.
Pandanus conoideus Lam. also known as the local name of Buah Merah, is an edible plant used traditionally as healthy food for daily life as natural multivitamins. The plant already reported curing some diseases together with pharmacological activities, including anticancer, antibacterial, antioxidant, etc. According to the phytochemical analysis, data in this study showed a correlation between their pharmacological activities with chemical constituents. Table 1 described that Buah Merah contained secondary metabolites of flavonoid, triterpenoid, and alkaloid, which distributed into different extracts according to their chemical properties, while the information of their bioactive constituent as antibacterial compounds against pathogenic oral bacteria was not yet reported.
According to preliminary of the antibacterial assay, Buah Merah extracts against E. faecalis showed that the ethyl acetate was the most active extract. Analysis of combination data of phytochemical screening and antibacterial activity of the extract in Tables 1 and 2, it has been noticed that ethyl acetate extract contains flavonoids compounds with antibacterial properties against E. faecalis ATCC 29212. This is an interesting finding because the antibacterial flavonoid from Buah Merah was not reported, while on the chemical structural aspect, it is not uncommon, because flavonoid is one part of the important secondary metabolites found in plants that show antimicrobial activity by causing various types of bacterial damage through several mechanisms such as degradation of bacteria cell walls, damage to the cytoplasmic membrane and due to waste of cellular material, cytoplasm coagulation and disruption on the ion/electrons membrane transport system. Any damage to the cellular surface causes a rupture in the cell surface, which leads to the output of vital cellular components and dangerous entry into bacterial cells [31].
Based on the selection of active extracts against E. faecalis ATCC 29212, the ethyl acetate extract was then subjected for separation and purification of their active antibacterial compounds by bioactivity guide technique and give four compounds 1-4. The structure elucidation of the active compounds was determined based on the measurement of UV, FTIR, NMR, and Mass spectroscopy. According to an analysis of the interpretation of their spectral data and compared to previous research, the chemical structures of the compounds were identified as flavonoid derivative of 1-2, as shown in Fig. (1).
Furthermore, four compounds isolated from ethyl acetate fraction were tested for their antibacterial properties resist E. faecalis ATCC 29212. The data in Table 3 indicated that compounds 1 and chlorhexidine had inhibition zones of 8.80 and 11.90 mm at 20000 and 2000 ppm with MIC and MBC values of 2500, 5000 ppm, respectively. The activity of compound 1 was correlated to its structure as flavonoid derivatives, a large group of secondary metabolites already isolated as antibacterial compounds from natural products [32]. On the other hand, compound 2, as well as compounds 3 and 4 are members of palmitic fatty acid groups, which were inactive and had no effect on E. faecalis
Various cell functions in the eukaryotic system are influ- enced by flavonoids. Although many studies are examining the mechanisms underlying the flavonoid antibacterial activity, they argue that different target compounds have different functions and target different bacterial cell components [33]. Some flavonoids antibacterial properties are: inhibiting DNA synthesis, ring B of flavonoid compounds interaction with nucleic acid-base structure via intercalation or hydrogen bonding, and inhibits DNA and RNA synthesis [34]. Flavonoids inhibit the function of cytoplasmic membranes. Mirzoeva et al. (1997) supported this and discovered that Quercetin increases membrane permeability in bacteria and leads to the disappearance of membrane integrity [35-38]. Recently, the researchers reported that flavonoid inhibits the MurA enzyme, which catalyzes the first committed step of peptidoglycan synthesis [39,40]. The results indicate that the flavonoid compound in Buah Merah has antibacterial properties against E. faecalis. The other important finding is the fact that Buah Merah is an edible fruit of a medicinal plant, as consequently, it was suggested that Buah Merah could be used to consume as an alternative natural antibacterial agent for the treatment of carries disease with low or no side effects.
CONCLUSION
Drug discovery research for new antimicrobial agents from herb plants is a potential challenge in the resolves of new drug candidates, especially to treat oral disease without negative side effects. The present study results indicate that Quercetin-3-methyl ether, which is a flavonoid as lead antibacterial compounds possess good antibacterial activity, with bioactive components, and can be used to treat dental caries. This finding suggested that Buah Merah’s extracts contain antibacterial flavonoids and effective resist pathogenic oral bacteria of E. faecalis ATCC 29212. The in vitro data is important to design further in vivo and in silico study to determine if the actual potency of the plant to tackle organisms causes infectious diseases and explain their mechanism actions drugs target interaction before clinical test steps. Discovery of a new antibacterial agent from promising of an edible plant may open the possibilities of finding new clinically effective antibacterial compounds against dental caries and other bacterial oral pathogens.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
None
HUMAN AND ANIMAL RIGHTS
No animals/ humans were used in this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS:
Supplement material contained ultraviolet, infrared, NMR, and mass spectra can be found on http://repository.unpad. ac.id/frontdoor/index/index/docld/200006.
FUNDING
Research Grant of Academic Leadership Grant (ALG) 2019 with Grant No. 1419/UN6.RKT/LT/2019 from Universitas Padjadjaran, Bandung, Indonesia.
CONFLICT OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The researchers are grateful to acknowledge Universitas Padjadjaran for Research facilities.


