All published articles of this journal are available on ScienceDirect.
Parents’ Perceptions of Breathing Pattern Changes, Sleep Quality, and Fatigue in Children after Rapid Maxillary Expansion: A Survey and Case Series Study
Abstract
Background:
Rapid Maxillary Expansion (RME) aims to re-establish balance between the widths of the jaws. It is mainly utilized to treat skeletal and dental manifestations associated with transverse maxillary constriction and to improve facial structures involving the nasal cavity.
Objectives:
This study aimed to investigate parents’ perceptions of breathing pattern changes after their child had undergone RME and the associated effects on sleep quality and fatigue. We also evaluated nasal cavity changes in three dimensions in six randomly selected patients.
Methods:
Ninety-one children aged 5-13 years with transverse maxillary deficiency and no major systemic diseases or syndromes were recruited. Their parents completed a 16-item questionnaire pre-treatment and 6 months post-treatment. The questionnaire included items pertaining to changes in (1) sleep apnea and breathing patterns, (2) sleep quality and fatigue, and (3) behavior. The cone beam computed tomography scans from six randomly chosen patients were also subjected to stereolithographic reconstruction of the midface pre-RME and post-RME.
Results:
Responses in the three domains exhibited good reliability. Significant improvements were observed in 59% of the items post vs. pre-RME. The overall rates of dry mouth in the morning, snoring half of the time, and heavy breathing decreased by ≥30%. The percentage change in headache in the morning, snoring loudly, and snoring half of the time was >80%. In addition, in the series of six cases, the mean difference in nasal cavity area post-RME was 4.1 mm2.
Conclusion:
Post-RME, parents perceived that their children exhibited improved behavior and were less fatigued during the day. Enhanced sleep quality and breathing patterns were also observed, but to a lesser extent.
1. INTRODUCTION
Rapid Maxillary Expansion (RME), also known as palatal expansion, is a surgical procedure that splits the mid-palatal suture and moves the maxillary shelves away from each other to re-establish balance between the widths of the jaws. The procedure was introduced by Angell in 1860 [1], and is main utilized to treat dental manifestations in patients who have transverse maxillary constriction, i.e., lateral discrepancies that result in unilateral or bilateral posterior crossbite and crowding [2]. Further, RME has been used as an interceptive measure to treat many pediatric medical conditions [3], such as Obstructive Sleep Apnea (OSA) [4-7], sleep-disordered breathing [8, 9], and nocturnal enuresis [9, 10]. Numerous studies have shown that the advantages of RME extend beyond the maxillary arch and dental occlusion, and include beneficial effects on adjacent facial structures such as the nasal cavity, breathing, and quality of life [8, 11-13].
A reliable and effective method for evaluating the treatment of maxillary palatal constriction is Cone Beam Computed Tomography (CBCT) [14]. Indeed, CBCT is considered the gold standard modality for studying complex changes in the anatomy of the airway [11] due to its relatively low cost, low radiation doses, short scan times, and its overall accuracy [15]. Using CBCT or cephalometric analyses, several reports have noted changes in the nasal airways that are attributable to RME [2, 16]. However, there is still some controversy in the literature concerning RME’s positive effects on the nasal cavity and airway [4, 17-19]. Doruk et al. [13], evaluated nasal airways after RME and reported that 59% of patients in their study exhibited improved nasal breathing, while 41% reported no change. Similarly, in a study reported by Oliveira De Felippe et al. [19], 61.3% of subjects reported improvement after RME and enhanced quality of life. In contrast, Langer et al. [17], reported that the effects of RME on breathing were not significant in the short term and that while significant improvements were observed after 30 months, these may have been due to ongoing facial growth. Likewise, in a prospective longitudinal study reported by Magnusson et al. [12], in 2010, while there were significant improvements in nasal obstruction after RME, these improvements only persisted in the long term in subjects with initial nasal obstruction. Hence, the exact effect of RME on the structure and function of the nasal airways remain unclear.
Therefore, the aims current study were to investigate subjective parental perceptions of breathing pattern changes in their children after RME and to determine the effects of this therapy on sleep quality and fatigue, as well as behavior, via a questionnaire. We also evaluated nasal airway changes in three dimensions using CBCT in six randomly selected patients.
2. MATERIALS AND METHODS
The current prospective survey-based investigation was a qualitative observational study. We also assessed the nasal cavity changes in three dimensions using CBCT in six randomly selected patients. A total of 116 patients were consecutively enrolled from a private practice in Pittsburgh, Pennsylvania, USA. Patients were selected in accordance with predetermined inclusion criteria, which included being of American Society of Anesthesiologists (ASA) 1 status, aged 5-13 years, exhibiting transverse maxillary deficiency manifesting clinically as bilateral posterior crossbite, and parental perception of breathing difficulties. Patients were only included in the study if they satisfied all of the inclusion criteria and their parents provided written informed consent permitting their inclusion.
The patients’ parents or legal guardians were asked to complete the Pediatric Sleep Questionnaire (PSQ) survey [20] consisting of 16 questions to be answered by either “yes” or “no” (Appendix). The survey was found to be reliable and was validated previously by Chervin et al., [21]. The license to use the survey was obtained via Michigan University, agreement number 1116-umish.
Assessments of parents’ observations about changes after treatment were divided into the following three domains: (1) sleep apnea symptoms and breathing patterns, (2) sleep quality and fatigue, and (3) behavioral changes. Each patient underwent pre-operative and post-operative assessments including a diagnostic cast mounted on a semi-adjustable articulator, intra-oral and extra-oral photographs, and CBCT.
Patients were treated with RME using a bonded Hyrax expander (Fig. 1). They were instructed to turn the expander key so that the total amount of expansion achieved in 2 weeks was 8-11 mm, with total retention duration of 1 year. Follow-up visits were conducted at monthly intervals. At the final follow-up visit at 1 year, the patients’ guardians were asked to complete the same survey questionnaire that they had completed pre-treatment. The guardians of only 91/116 patients completed the survey the second time.
To analyze the data, we summarized the rates of each symptom the parents reported within each domain (sleep apnea symptoms and breathing patterns, sleep quality and fatigue, and behavioral changes) before and after RME, and McNemar’s test was used to compare the data due to the paired study design. We also calculated the absolute difference in the pre-RME and post-RME rates and the relative difference, i.e., the percentage change after RME. All Analyses were conducted using SAS [22], version 9.4 (SAS Institute, Cary, NC), with statistical significance set at P < 0.05.
We then calculated the mean number of symptoms parents reported within each domain before and after RME and compared them using a paired t-test. We assessed the internal reliability of each domain using Cronbach’s alpha.
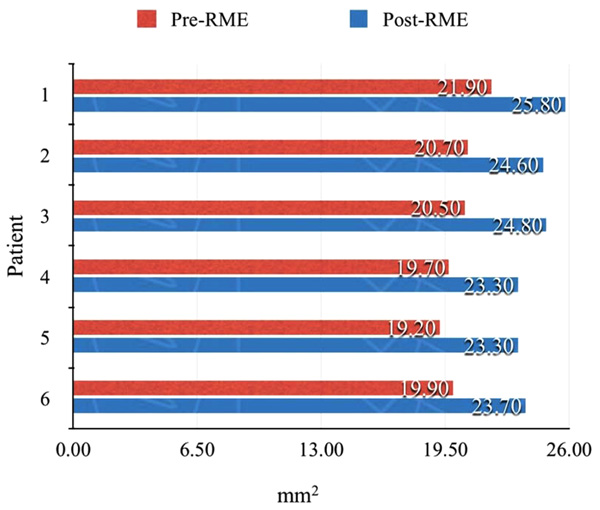
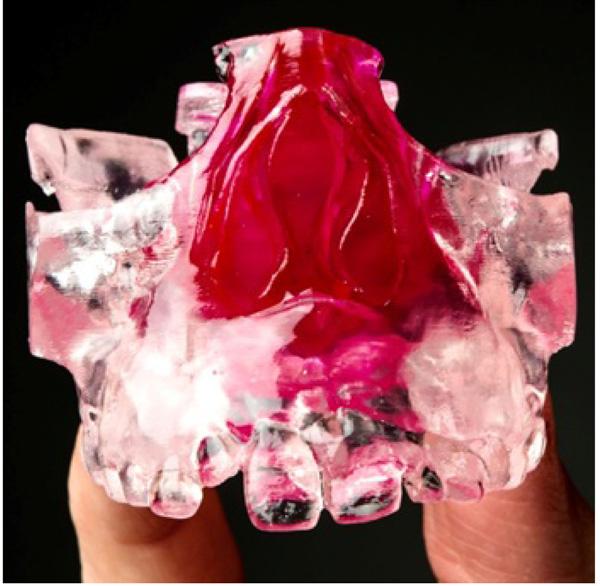
In addition, a series of six patients had pre-treatment and post-treatment CBCT scans printed Three-Dimensional (3D) models, in Colorado using 3D Systems, to measure changes in the nasal cavity using an orthodontic ruler. The measurement point was from the base of the inferior concha to the soft tissue lining of the nasal septum. Measurements were also made on both pre and post CBCT scans using 3D Systems Medical Modeling Software.
3. RESULTS
The guardians of 91 patients (49 boys [53.9%]) completed the survey. The mean (Standard Deviation [SD]) age was 7.6 (5.0) years pre-treatment and 8.2 (5.0) years post-treatment.
The perceived sleep apnea symptoms and breathing patterns domain included nine items, and both pre-treatment and post-treatment data were obtained from the guardians of 88 patients for this domain. The pre-treatment Cronbach’s alpha was 0.61, indicating acceptable reliability, but it was 0.41 post-treatment, indicating poor reliability. The mean (SD) number of symptoms reported by patients’ guardians was 3.14 (1.93) symptoms pre-treatment and 1.26 (1.21) symptoms post-treatment (p < 0.001, paired t-test), with the mean (SD) difference being 1.91 (1.90).
The perceived sleep quality and fatigue domain included seven items, and both pre-treatment and post-treatment data were obtained from the guardians of 89 patients for this domain. The respective pre-treatment and post-treatment Cronbach’s alpha values were 0.66 and 0.53, indicating acceptable reliability at both time points. The mean (SD) number of symptoms patients’ guardians reported was 1.21 (1.45) symptoms pre-treatment and 0.43 (0.84) symptoms post-treatment (p < 0.001, paired t-test), with the mean (SD) difference being 0.79 (1.47).

The perceived behavioral changes domain included six items, and both pre-treatment and post-treatment data were obtained from the guardians of 88 patients for this domain. Cronbach’s alpha was 0.85 both pre-treatment and post-treatment, indicating good reliability at both time points. Patients’ guardians reported a mean (SD) number of 2.07 (2.16) symptoms pre-treatment and 1.64 (2.01) symptoms post-treatment (p = 0.02, paired t-test), with the mean (SD) difference being 0.44 (1.63).
The respective percentages of subjects with adenoids and/or tonsils who reported sleepiness during the day pre-RME and post-RME were 2% and 1%, and the respective corresponding percentages in subjects with excised adenoids and/or tonsils were 7% and 3%. The respective percentages of subjects with adenoids and/or tonsils who reported a stoppage in breathing pre-RME and post-RME were 12% and 2%, and the respective corresponding percentages in subjects with excised adenoids and/or tonsils were 10% and 6%.
As shown in Table 1, 13 of the 22 items (59%, approximately two thirds) improved significantly after RME. Reductions of 30% or more were noted for dry mouth in the morning (62% vs. 32%), snoring half of the time (37% vs. 7%), and heavy breathing (62% vs. 24%). The relative difference, i.e., percentage change after RME, was >80% for headache in the morning (7% vs. 0%, percentage change = 100%), snoring loudly (27% vs. 4%, percentage change = 87%), and snoring half of the time (37% vs. 7%, percentage change = 81%). In the six consecutive patients from which 3D models were derived, the improvement ranged from 3.6 - 4.4 mm2 (Figs. 2-4).
4. DISCUSSION
The current literature is rich in studies that tackle the effectiveness of RME in improving sleep breathing patterns through nasal cavity widening and reducing airflow resistance. However, evidence of RME’s effects on a child’s sleep apnea symptoms and breathing patterns, sleep quality and fatigue, and behavioral changes are less abundant. Three main findings regarding these factors were identified in the current study.
First, the parents of 96% of patients (87/91) reported significant improvements in sleep apnea, a finding that is in accordance with those of Doruk et al. [13], demonstrating that 59% of patients reported improved breathing after RME, and Magnusson et al. [12], whereby a significant improvement in subjectively evaluated nasal obstruction was noted after 3 months. Moreover, this research’s finding of significant improvements in sleep apnea post-RME is in line with the findings of a literature review by McNamara et al. [4], that collected data about RME and its effects on oral and general health. The review concluded that, disregarding the long-term stability, RME can widen the nasal cavity base and increase its volume, thereby improving breathing disorders including OSA. However, a study by Langer et al. [17], concluded that contrary to previous reports, the effects of RME on the nasopharyngeal area were insignificant in the short term, noting that although the nasopharyngeal area increased significantly by 30 months, this may have been due to ongoing facial growth. Nonetheless, given to the shorter follow up period of the current study, it can be said that the results achieved were purely due to RME rather than normal growth or development.
The second main finding was that the parents of 98% of the patients (89/91) reported less fatigue and better sleep quality post-RME.
The third main finding was that 97% of the patients (88/91) reported improvements in behavioral changes and hyperactivity.
The most recent research project conducted in 2018 by Ashok et al. [8], studying the effects of RME on sleep characteristics in children revealed that RME enhances sleep quality and increases children’s sleeping hours. This finding is also reflected in the current study as 98% of the parents reported improved sleep quality post RME.

| Symptoms | Pre-RME | Post-RME |
Absolute Difference in Rates between
Pre-RME and Post-RME |
Relative Difference, % Change after RME | McNemar’s Test |
|---|---|---|---|---|---|
| - | N (%) | N (%) | % | % | p value |
| Sleep Apnea Symptoms and Breathing Patterns (9 items) | - | - | - | - | - |
| Snoring half of the time | 32 (37.2) | 6 (7.0) | 30.2 | 81.2 | <0.0001 |
| Always snoring | 19 (22.4) | 1 (11.2) | 11.2 | 50.0 | <0.0001 |
| Snore loudly | 23 (27.4) | 3 (3.6) | 23.8 | 86.9 | <0.0001 |
| Heavy breathing | 51 (62.2) | 20 (24.4) | 37.8 | 60.8 | <0.0001 |
| Trouble breathing | 13 (15.5) | 4 (4.8) | 10.7 | 69.0 | <0.0001 |
| Stop breathing | 9 (10.2) | 4 (4.6) | 5.6 | 54.9 | 0.096 |
| Breathing through the mouth | 54 (63.5) | 37 (43.5) | 20 | 31.5 | 0.003 |
| Dry mouth in the morning | 52 (61.9) | 27 (32.1) | 29.8 | 48.1 | <0.0001 |
| Wet the bed | 18 (20.2) | 10 (11.2) | 9 | 44.6 | 0.01 |
| Sleep Quality and Fatigue (7 items) | - | - | - | - | - |
| Feeling unrefreshed | 33 (37.1) | 11 (12.4) | 24.7 | 66.6 | <0.0001 |
| Sleepiness during the day | 22 (24.4) | 8 (8.9) | 15.5 | 63.5 | 0.002 |
| Teacher comment on sleepiness | 9 (9.9) | 2 (2.2) | 7.7 | 77.8 | 0.02 |
| Hard to wake up in the morning | 29 (31.9) | 13 (14.3) | 17.6 | 55.2 | 0.002 |
| Headache in the morning | 6 (6.7) | 0 | 6.7 | 100.0 | / |
| Normal growth rate | 8 (8.9) | 3 (3.3) | 5.6 | 62.9 | 0.096 |
| Overweight | 3 (3.3) | 1 (1.1) | 2.2 | 66.7 | 0.16 |
| Behavioral Changes (6 items) | - | - | - | - | - |
| Listens when spoken to directly | 24 (27.6) | 18 (20.7) | 6.9 | 25.0 | 0.18 |
| Difficulty organizing tasks and activities | 25 (27.8) | 15 (16.7) | 11.1 | 39.9 | 0.02 |
| Easily distracted | 36 (41.4) | 29 (33.3) | 8.1 | 19.6 | 0.09 |
| Fidgets with hands and feet or squirms in seat | 38 (42.7) | 34 (38.2) | 4.5 | 10.5 | 0.32 |
| Child on the go or driven by motor | 27 (30.3) | 23 (25.8) | 4.5 | 14.9 | 0.29 |
| Interrupts or intrudes on others | 30 (35.3) | 22 (25.9) | 9.4 | 26.6 | 0.06 |
The case series in which objective measurements were performed on the printed 3D models of CBCT images confirmed the increase in the nasal cavity size identified in the subjective surveys. CBCT is an accurate tool for viewing and collecting information regarding the Oro-nasal structures. In addition to this, by employing the 3D capabilities of additive manufacturing, these 3D models are now an additional medium through which health care professionals can clearly illustrate and share the effects of RME on Oro-nasal structures.
The present study had several limitations. First, some parents did not complete the survey post-RME, did not return the survey, or chose not to participate in the study. Second, Although this study employed a convenience sample, it utilized a single operator expansion protocol. Third, we cannot eliminate the potential bias inherent in this type of study. Fourth, since this was a survey-based study, the patients’ parents may have tended to answer “yes” in parts of the survey alluding to improvements after treatment. Fifth, although we cannot draw a conclusion based on the case series, 3D printing is an advanced technology that is being increasingly used to measure physical changes in the upper airway. Future studies should use this approach given its advantages over rhinomanometry, one of which is giving a physical measure of the actual enlargement, for measuring breathing improvements after treatment [4]. Finally, a valid control group could not be included due to the ethical obligation to provide the necessary treatment to all patients.
CONCLUSION
In sum, parents’ subjective perceptions were that their children exhibited improved behavioral changes and less fatigue during the day post-RME. Parents also noticed the enhanced quality of sleep and breathing patterns after RME, but to a lesser extent.
LIST OF ABBREVIATIONS
| 3D | = Three-Dimensional |
| CBCT | = Cone Beam Computed Tomography |
| OSA | = Obstructive Sleep Apnea |
| RME | = Rapid Maxillary Expansion |
| SD | = Standard Deviation |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study is approved by ethical IRB committee from King Abdulaziz University. Approval date 23/04/2017, Number: 030-03-2017.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008.
CONSENT FOR PUBLICATION
Consent was obtained from the parents or guardians of all children for this study to be published.
FUNDING
None.
CONFLICTS OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank Ms.Melissa Herbinko for her time and effort.
APPENDIX
Questionnaire for Parents of Patients Before/After Rapid Maxillary Expansion
Patient’s Name: _________________________________
Patient’s Date of Birth: _____/_____/_______
Patient’s Age: __________
Patient’s Sex: M F
Please Circle the Answer that Best Describes your Child.
- While sleeping, does your child…
- Have you ever seen your child stop breathing during the night?
- Yes No
- Does your child…
- Does your child…
- Has a teacher or other supervisor commented that your child appears sleepy during the day?
- Yes No
- Is it hard to wake your child up in the morning?
- Yes No
- Does your child wake up with headaches in the morning?
- Yes No
- Did your child stop growing at a normal rate at any time since birth?
- Yes No
- Is your child overweight?
- Yes No
- This child often does not seem to listen when spoken to directly.
- Yes No
- This child often has difficulty organizing tasks and activities.
- Yes No
- This child is often easily distracted by extraneous stimuli.
- Yes No
- This child often fidgets with hands or feet or squirms in seat.
- Yes No
- This child is often “on the go” or often acts as if “driven by a motor.”
- Yes No
- This child often interrupts or intrudes on others (e.g. butts into conversations or games)
- Yes No
- Have your child’s tonsils/adenoids been removed?
- Yes No
And if so, when?___________________________
THANK-YOU!
Do you consent to the collection of your child’s breathing data for use in scientific research and analysis? If so, please sign below. Thank you.
Parent/Guardian Signature: ______________________ Date:_____/_____/______


