All published articles of this journal are available on ScienceDirect.
Efficacy of In-office Bleaching on Microhardness of Permanent Teeth with Antioxidant Re-hardening
Abstract
Background:
Bleaching procedures affect surface enamel structure and decrease its bonding ability to resin composite restorative materials. The application of re-hardening materials to bleached enamel surfaces may prevent this decrease in micro-hardness.
Objective:
This in-vitro study aims to evaluate the surface micro hardness of human teeth enamel subjected to bleaching with Zoom Advanced Power 2 AP (Phillips, USA), and Opalescence Boost (Ultradent, USA) and compare the re-hardening effects of 10% Sodium Ascorbate, 2% acidulated phosphate fluoride gel, and a 5% Potassium nitrate 0.22% Sodium Fluoride + Calcium Nitrate gel.
Methods:
Ninety human third molar teeth were used. The specimens were randomly assigned to 5 groups. After the bleaching procedure, the specimens were treated with APF, Sodium Ascorbate or Relief gel as re-hardening agents with 30 teeth in each group. Enamel micro-hardness was measured with Vickers Micro-hardness Tester. The data were evaluated with Kolmogorov-Simirnov, one-way ANOVA, Dunnett’s test, post-hoc Tukey and T-tests.
Results:
Statistical analysis revealed no significant differences among initial enamel groups’ micro-hardness values (P>.05); however, significant differences occurred between initial and after bleaching treatment group value for G3 (P< .05). After re-hardening, only the Sodium Ascorbate group showed a statistically significant increase with hardness values (P< .05) for G4 and G5.
Conclusion:
Bleaching treatment conducted with light had no adverse effect on enamel micro-hardness. Sodium Ascorbate can be useful after bleaching to change the adverse effects of bonding on the enamel.
1. INTRODUCTION
The current patient population is more demanding of restorations that preserve the aesthetic, and provide aesthetically preferred tooth color [1-4]. After bleaching, new composite restorations may be made, or already present composites may need to be changed in order to match the new tooth color. However, renewal of old composite restorations is usually not made immediately after bleaching procedures, due to the negative effects of bleaching on the bonding of composites [4].
There are a number of protocols that have been described in the literature for bleaching vital teeth [1, 4]. Among these methods, in-office bleaching technique has some advantages over other techniques including better clinical control, avoidance of soft-tissue exposure and material ingestion, reduced total treatment time and greater potential for immediate results that may increase patient satisfaction and motivation [2]. Furthermore, patients may prefer bleaching by a dental professional because of better result, rapid procedure and less active participation part of the professional [3].
In-office bleaching commonly involves the Application of Hydrogen Peroxide (HP) (usually 35%) and a light source to accelerate bleaching effect, which is called “power-bleaching” [1]. Proponents of power bleaching claim to reduce the total in-office bleaching time by energizing the bleaching material using various light sources [2].
Although, mostly they are reversible with the degradation of bleaching agent, the pH becomes acidic and bleaching can cause some alterations on the hard dental tissues [4, 5]. Alterations of enamel properties like decreased micro-hardness, mineral loss, increased roughness and porosity have been reported [5, 6]. However, the results reported in the literature are controversial since some other studies did not find any significant difference in enamel after bleaching [7-10].
Another important complication besides possible enamel hardness reduction is bleaching agents, which include Hydrogen (HP) and Carbamide (CP) peroxide, which can decrease the bond strength of composites on acid-etched enamel when performed immediately after the bleaching treatment [8, 9]. It is believed that this happens because residual oxygen and peroxide remain on the tooth surface or inside the tooth after bleaching treatment and prevent complete polymerization of adhesive resin. The lower bond strengths of bleached enamel and dentin are a result of the oxidative process caused by bleaching agents [4-9].
Nevertheless, it was observed that the use of anti-oxidant agents before the bonding process could reverse the compromised bonding to bleach enamel [4-14]. Thus, with this method, the restorative procedures could be performed immediately after the bleaching procedure. Ascorbic acid and its salts are well-known antioxidants and are capable of reducing a variety of oxidative compounds, especially free radicals [11]. Since ascorbic acid (vitamin C) and its salts are non-toxic and are widely used in the food industry as antioxidants, it is unlikely that their intra-oral use will result in any adverse biological effects or clinical hazards [11].
Oskoee et al. [4] stated that electrochemical studies and topographical techniques reveal that Sodium Ascorbate is absorbed by flat polymer surfaces and hydroxyapatite crystals. Furthermore, it does not have any negative effect on the bonding procedure of the composite like other re-hardeners. Therefore, it may thus be a viable alternative for the patients who require re-hardening of the tooth enamel after bleaching and before composite restorative treatment.
The purpose of this in vitro study was to compare the effects of UV power bleaching using light and high concentration bleaching agents on tooth enamel micro-hardness, and the effects of an antioxidant on bleached enamel micro-hardness compared to conventional re-hardeners. The first null hypothesis of this study is that the HP bleaching agent with UV light does not affect the enamel micro-hardness. The second null hypothesis of this study was that antioxidant treatment does not increase bleached enamel micro-hardness.
2. MATERIALS AND METHODS
Ninety recently extracted intact human third molar teeth were used in this study. Immediately after extraction, the teeth were kept in 10% formaldehyde solution. The study was approved by the University Institutional Ethical Committee.
2.1. Preparation of Samples
Each tooth was scraped of detritus, tissue and deposits with a surgical blade. The roots were removed approximately 1-2 mm apical to the cemento-enamel junction and the teeth were sectioned into buccal and lingual halves, using a slow speed saw with a double-sided diamond disc (Isomet Buehler Ltd, Chicago, IL USA) under water irrigation.
All the teeth were kept in non-ionized distilled water during all phases of the experiment to prevent dehydration. Acetate molds in 2 mm diameter were prepared and the tooth specimens were embedded individually in self-curing acrylic resin. Middle third of the facial enamel of specimens were slightly flattened and polished, and used for the study. The specimens were randomly divided into 5 groups (n: 18) according to the bleaching agents. The active ingredients of all materials are given in Table 1.
| Bleaching Material | Manufacturer | Active Ingredient |
|---|---|---|
| Zoom 2 Gel | Discus Dental, USA | 25% Hydrogen peroxide, KNO3, ACP |
| Zoom AP Light | Discus Dental, USA | Sodium free, 25 W short arc halide lamp, 350-600 nm |
| Opalescence Boost Gel | Ultradent Products Inc., USA | 38% Hydrogen peroxide, NaF |
2.2. Bleaching Procedure
Before the bleaching procedure, specimens were gently dried for moisture control. Each specimen group was subjected to the bleaching treatments thus obtaining a uniform gel layer with 2.5 mm thickness for three sequences of fifteen minutes:
- G1: Placebo gel and Zoom AP light. (Colorless glycerin-based placebo gel was used to prevent dehydration)
- G2: Zoom 2 25% hydrogen peroxide in-office bleaching gel
- G3: Zoom 2 25% hydrogen peroxide in-office bleaching gel and Zoom AP light,
- G4: Opalescence Boost 38% hydrogen peroxide in-office bleaching gel,
- G5: Control (no light no gel).
2.3. Re-hardening Procedure
The specimens were randomly distributed in 5 groups each. These groups included 3 surface treatment subgroups after bleaching for re-hardening procedure: 2% acidulated phosphate fluoride (Topex APF gel, Sultan Healthcare, USA) (subgroup APF; n: 6), a 5% Potassium nitrate 0.22% Sodium Fluoride + Calcium Nitrate gel (Relief ACP Gel, Discus Dental, USA) (subgroup RG; n: 6), and 10% Sodium Ascorbate solution (subgroup SA; n: 6).
APF was applied to enamel surfaces for 60 seconds, Relief ACP gel was applied for 5 minutes and 10% Sodium Ascorbate (pH 7.4) was applied on the enamel surfaces for 10 minutes. For SA subgroup, 1 ml SA solution was applied on the sample for ten minutes with a syringe in order to keep the enamel surface wet.
2.4. Micro-hardness Measurement
Micro-hardness measurements were made before and after bleaching, and post treatment on each sample using a Vickers micro-hardness (VHN) tester (HMV-II, Shimadzu, Japan), with a minimum diagonal length measurement accuracy of 0.01m in x40 magnification. The indentations were made on the flattened middle third top surface of each specimen using 300 g loads and a dwell time of 20 seconds. Three measurements were made from each sample, and the values were averaged to produce one value for each sequence without leaving any redundant data out of the data pool.
3. RESULTS
3.1. Micro-hardness Results
3.1.1. Pre and Post Bleaching
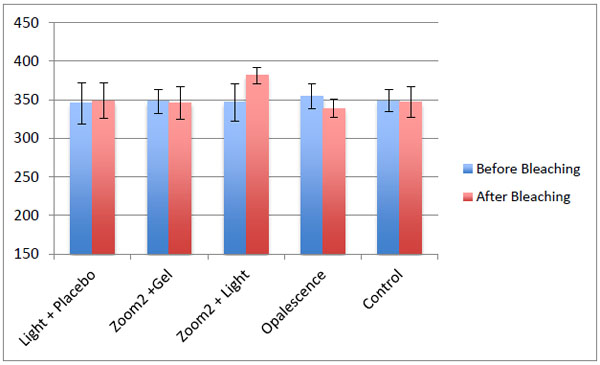
The mean and standard deviations of the pre and post bleaching enamel micro-hardness values of samples are listed in Fig. (1).
Before bleaching, no significant difference was found on VHN between 5 groups and control groups (p>0, 05). Bleaching changes were found in the results of the 5 experimental groups. Specimens treated with placebo gel and Zoom UV Light (G1) and Zoom 2 & Zoom AP UV light (G3) showed an increase in VHNs. Specimens treated with Zoom 2 & Zoom AP UV light (G3) showed a statistically significant increase in VHN compared to the control group (p<0,05). Specimens treated with Zoom 2 25% hydrogen peroxide (G2) Opalescent boost (G4) and Control (G5) showed a small decrease and these differences were not statistically significant compared to the control groups (p>0.05).
3.2. Re-hardening Results
The mean and standard deviations of changes in micro-hardness values of re-hardening agents are shown in Figs. (2-4).

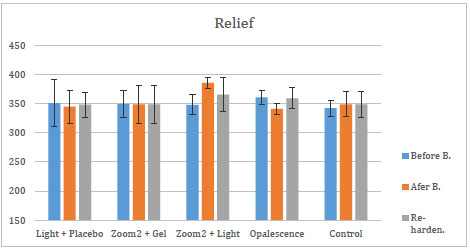
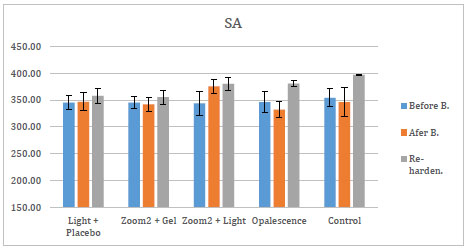
After the application of these three re-hardening agents, SA showed an increase in micro-hardness for all groups. The efficacy of those three agents was assessed over each of the five bleaching groups. After the application of APF and Relief, there was no statistical change in the groups. After the application of SA, the increase in micro-hardness of G4 and G5 was significant (p<0, 05) compared to micro-hardness values of bleaching treatment of the same groups.
4. DISCUSSION
In general, Sodium Ascorbate application provided higher re-hardening values compared to acidulated phosphate fluoride gel and Relief gel. According to the literature, the effects of bleaching agents are related to exposure time, type and concentration of the active ingredient [5]. In this in-vitro study, the manufacturer’s directions for the product application time and method were followed exactly, in order to mimic clinical conditions and clinical material’s effects while conducting evaluations.
All human teeth enamel samples’ initial micro-hardness values were measured before procedures, and no significant differences were found among all the control groups. Heat emitted from certain light sources may skew the observed whiteness of the teeth, and possibly affect the micro-hardness measurements, which were therefore avoided in this study. Zoom Advanced Power whitening system has a UV light source, which is a Sodium-free, 25W, short-arc halide lamp, emitting light between 350 and 600 nm wavelength. This lamp is similar to solarium lamps and does not produce heat as a side effect; instead, Zoom 2 gel chemistry is based on Photo-Fenton reaction. In this chemical reaction, Fe2+ can react with H2O2 to produce Fe3+ and hydroxyl radicals (-OH) which are powerful oxidants which can subsequently react rapidly with aromatic compounds [3, 15].
For the evaluation of the surfaces, Vickers micro-hardness tester and Vickers Hardness Numbers (VHN) were used which are similar to many studies [5, 16-18]. Initial VHNs were measured with 300 g load and a dwell time of 20 seconds similar to a previous study and the results were ranged similar to KHNs in some studies [19-21]. On the other hand, it is known that micro-hardness measurement procedure requires flattening to provide a more uniform enamel surface to make indentation more precise [21, 22]. This procedure removes superficial, highly mineralized, prismless enamel surface layer, which is more resistant to demineralization. This may lead to unreliable results in evaluating the adverse effects of peroxide-containing bleaching products on dental tissues.
The results of this study indicated that a small increase was shown in Placebo gel and ultraviolet light (G1). UV light consequently reacts with Fe3+ to create Fe2+ (2) and these series of reactions occur cyclically, leading to the formation of hydroxyl radicals continuously, which results in whiter teeth [15, 16]. This may cause differences in superficial mineral content, although it is possible that the light itself cannot cause demineralization or remineralization. After eruption, the tooth enamel continues to mineralize in saliva before it reaches its maturity. For newly extracted human third molars, a possible hardness during the storage period in the distilled water for testing may be a possible cause of the increase in hardness [21].
Similar to the previously discussed placebo gel and utraviolet light group, an increase in enamel microhardness values was observed in Zoom 2 25% hydrogen peroxide and UV Light (G3) group and this difference was statistically significant. The increased microhardness values in this group may be attributed to the inclusion of Amorphous Calcium Phosphate (ACP). The results of this study are partly in agreement with the study conducted by Polydorou et al., [8]. which reported an increase in micro-hardness after bleaching in all of the three test groups; Zoom 2 & Zoom 2 Light unit, Opalescence Xtra Boost, and Easywhite Ready & plasma light unit, except the result of the Opalescence Boost group. In this study, this product has shown a decrease in micro-hardness which is not statistically significant. The difference between the two studies may be attributed to different application times or the method of application of the same agents.
Alternatively, as de Oliveira and others [23] reported that bleaching could even significantly increase enamel microhardness during in vitro bleaching treatment; the increase in microhardness found in their study may be attributed to the bleaching agent that was used. The unique composition of the bleaching materials used could have an effect on the results. Similarly, according to Justino et al., [24], the experimental design may affect the results [6, 25].
A decrease, which is not statistically significant, was observed in Zoom 2.25% hydrogen peroxide (G2) and Opalescence Boost 38% hydrogen peroxide (G4), which had no ACP in itx [25]. A clinical study showed that ACP compounds in a carbonate solution crystallize to form hydroxyapatite [26]. Opalescence Boost includes Sodium fluoride, which is added to counterbalance the demineralization effect of peroxides. The other possible reason may be the increased peroxide concentration (38%), which may have lead to a decrease in micro-hardness. There was a difference especially between high concentration Opalescence Boost group and other groups. In summary, bleaching agents in conjunction with specific experimental parameters and methodology may yield different micro-hardness results during testing.
Studies have reported that the addition of Calcium, Fluoride, APF, ACP, n-HAP, KF desensitizing agent, potassium nitrate, their combinations, and antioxidants in bleaching agents as a re-mineralizing agent or their usage after bleaching procedures, preventing changes in enamel demineralization micro-hardness and surface morphology without reducing the bleaching efficacy in-vitro. Some other procedures like phototherapy and surface removal were effective as well [27-31]. But there was not any study that compared the conventional re-mineralizing agents, desensitizers and antioxidants directly.
Lewinstein et al., [22] reported a light reduction in enamel surface micro-hardness following 35% hydrogen peroxide or 35% carbamide peroxide treatments on human enamel, which was completely reversed when treated with a 0.05% fluoride solution. Da Costa [25] used 1,23% APF gel after bleaching and reported that APF gel showed a significant increase when compared to groups that did not receive fluoride. Chen et al., [19] showed that fluoridated bleaching gels preserve enamel hardness more effectively than fluoridation after bleaching.
Bittencourt et al., [32] evaluated the influence of fluoride delivery and restoration time point on the micro-shear bond strength (mSBS) and degree of conversion (DC) values of an adhesive applied on bleached enamel. Groups were HP: 35% hydrogen peroxide, HPF: HP + 1.23% Sodium fluoride application and PF: 38% HP with F. Researchers stated that only the use of a fluoridated in-office bleaching agent (Opalescence Boost PF) proved to be effective in immediately reversing the side effects of low mSBS and DC values when in-office bleaching was used.
In the present study, APF, SA and ACP solutions were used for re-hardening of the specimens. Sodium Ascorbate showed an increase in micro-hardness values of all bleaching material groups. The increase may be due to its antioxidant effect and effects on enamel components. It is possible that the increase in surface micro-hardness subsequent to the use of 10% Sodium Ascorbate is related to the enamel surface adsorption of this agent.
APF and ACP showed slight increase but it was not statistically significant for all bleaching materials. But all of these products except antioxidants are not found as solutions to increase the bonding performance of enamel on composite [19, 30].
It should be stated that for bleaching patients, if restorative follow-up procedures are necessary, then Sodium Ascorbate may be a preferable alternative to fluoride and other current re-hardeners.
CONCLUSION
Within the limitations of this study, the light-activated bleaching does not appear to show damage to the enamel surface.
High concentration of HP (38% hydrogen peroxide) was more detrimental to the enamel surface but not significantly.
Sodium Ascorbate is found to be more effective in re-hardening the bleached teeth than ACP and APF solutions.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethical Committee of Gazi Universitesi Dishekimligi Fakultesi (University of Gazi, Faculty of Dentistry, Ankara, Turkey) with the project number: 03-2011-05.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this study are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


