All published articles of this journal are available on ScienceDirect.
Surgical Management of Granular Cell Tumor of the Orbit: Case Report and Literature Review
Abstract
Introduction:
Granular Cell Tumors (GCTs) of the orbit are rare-entity soft-tissue tumors, and few reports have been published in the literature. The treatment of the choice is total excision. Early diagnosis prior to surgery is valuable for the distinction of malignant from benign tumor.
Case presentation:
We report a case of a 55-year-old woman with a solitary slow-growing mass in the right orbit with the involvement of the rectus inferior muscle, and present a review of the recent literature. The lesion had a diameter of 1 cm and was noticed 2 years before the examination. Excisional biopsy confirmed the diagnosis of GCT. The tumor was resected through a retroseptal transconjunctival approach. The final histological examination revealed findings characteristic of GCT, including positive reaction for protein S-100, SOX10, and calcitonin and negative reaction for desmin, myogenin, Smooth Muscle Antigen (SMA), Melan-A, and HMB-45. There were no signs of malignancy in this sample. Disturbance of motility was not noted by the patient after surgery.
Conclusion:
GCT should be included in the differential diagnosis of intraorbital lesions, particularly those that involve the orbit muscles. A biopsy is recommended before surgical resection, to exclude malignancy and prevent radical resection.
1. INTRODUCTION
Granular Cell Tumor (GCT) is mostly a benign soft-tissue tumor with a Schwann cell phenotype that can arise in any site in the body [1]. The most common sites are arm, chest wall, and tongue [2]. GCT was described by Abrikossoff in 1926 as a tumor of the tongue with granular cells derived from striated muscle [3]. This tumor was initially called granular cell myoblastoma or myoblastic myoma due to the location. However, this terminology is now considered to be controversial due to the histogenesis of the tumor [3].
The occurrence of GCT in the orbit is very rare, and to date, only a few cases have been reported in the literature in English. GCT of the orbit presents as a slow-growing, solitary, well-demarcated unilateral lesion. Common clinical manifestations are swelling and pain, alone or in combination [1]. The condition is most common in women between the fourth and the sixth decades of life [4]. The presenting symptoms are diplopia, proptosis, and ocular dysmotility [4].
GCT should be considered in the differential diagnosis of orbital mass, especially when the extraocular muscles are affected [1, 4, 5]. Involvement of ocular muscles and proptosis has been reported in 84.6% of orbital GCT cases [6]. Several imaging modalities have been used in patients with GCT of the orbit, including Computed Tomography scan(CT-scan) and Magnetic Resonance Imaging (MRI) [7, 8]. Radiological examinations reveal information such as tumor size, extent of the tumor, and metastatic spread [9]. Positron Emission Tomography and Magnetic Resonance Imaging (PET/ MRI) are more accurate than CT for the description of tumor involvement and soft tissue invasion [10]. However, microscopic analysis of the tumor is regarded as the gold standard for distinguishing between malignant tumor and benign tumor [11]. Although GCTs are mostly benign tumors, Malignant Granular Cell Tumors (MGCTs) comprise approximately 1-3% of all granular cell tumors [5]. This type has an aggressive growth with a high rate of recurrence [5]. The histological differentiation of MGCTs from benign GCTs is crucial for treatment outcome. The most characteristic histological criteria for MGCT are mitotic activity, necrosis, increase in nuclear/cytoplasmic ratio, and pleomorphism [12].
Although GCT is a neoplasm derived from neural crest cells, many pathologists routinely employ immunohistochemical nuclear staining, such as for S-100 and Sox-10, for diagnostic purposes [13, 14]. The treatment of choice for orbital GCT is surgical excision. However, following surgery, the risk of diplopia is high due to damage to extraocular muscles [4, 5].
Here we present a case of orbital GCT with the involvement of the rectus inferior muscle, along with a review of the recent literature. The importance of excisional biopsy and immunohistochemical analysis for correct diagnosis and treatment strategies are also discussed in this article.
2. CASE PRESENTATION
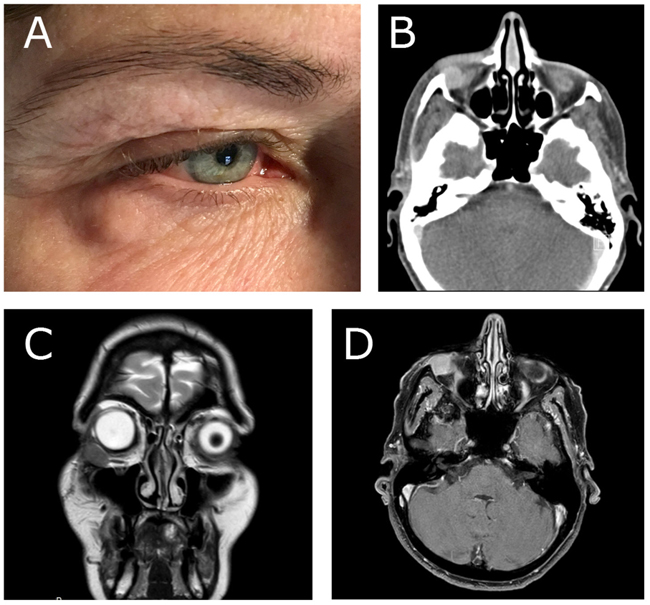
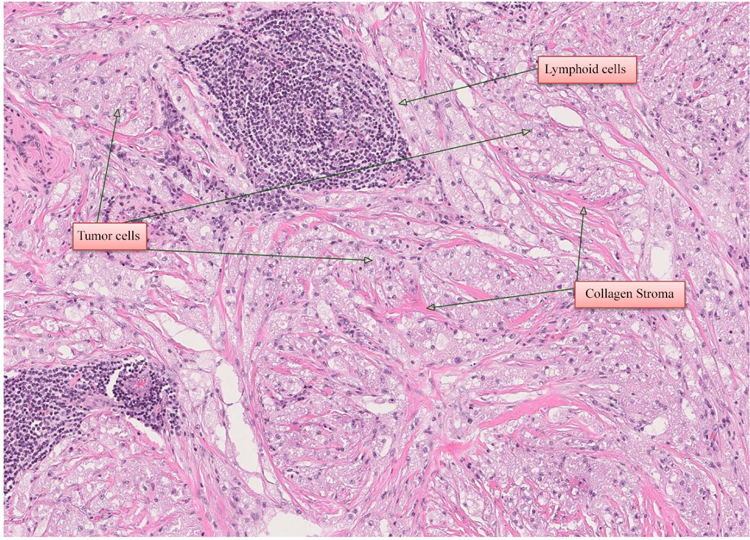
A 55-year-old woman was referred to the Department of Otorhinolaryngology, Head and Neck Surgery, Linköping University Hospital, Sweden, for evaluation of swelling at the lateral border of the right eye (Fig. 1a). No pain or visual disturbances were experienced by the patient. She had a 2-year history of swelling without any family history of malignancy or chronic disease. By palpation, a cartilaginous-like tumor with good motility and a smooth surface was felt at the conjunctive side of the right lower lid. Further investigation with slit-lamp biomicroscopy showed the presence of a subconjunctival mass adjacent to the right inferior rectus muscle. Force duction test did not reveal any restriction of muscle movement. Complete ophthalmological examination revealed intraocular pressure of 17 mm Hg on the right side and 15 mm Hg on the left side, with normal ocular motility. Visual acuity was 20/20 in both eyes. CT-scan showed a homogeneous soft-tissue mass of 14 × 14 × 8 mm in the right retrobulbar space, with bone erosion (Fig. 1b). Further MRI examination showed a solitary soft-tissue mass adjacent to the rectus inferior muscle (Figs. 1c & d). Excisional biopsy was performed, and histological examination identified the lesion as GCT (Fig. 2); H&E, magnification × 20). The histological pattern included lymphoid cells, fibrous stroma, and large polygonal eosinophilic cells with abundant granular cytoplasm and round nuclei. There were no signs of malignancy.
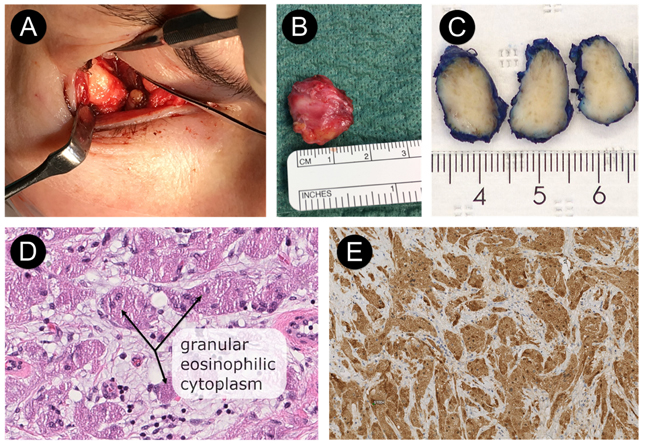
The tumor was resected through a transconjunctival approach using general anesthesia (Figs. 3a & 3b). The lower eyelid was everted using two stitches of 5-0 nylon through the tarsal plate. The incision was made using retroseptal approach through mucous membrane tissue in the right inferior fornix of the periorbital soft tissue (3 mm below the tarsus). The excised tumor measured 19 ×16 × 8 mm; it was firm and avascular (Figs. 3a & 3b). The tumor was infiltrated within the inferior rectus muscle, which made it difficult to excise completely. Thus, we had to remove the tumor with approximately 1 mm of the right lateral border of the inferior rectus muscle. Finally, the conjunctiva was closed with two 4-0 resorbable sutures.
Macroscopic appearance of the tumor revealed a gray-tan-colored to brownish mass measuring 19 × 16 × 8 mm (Fig. 3c). The specimen was fixed in 10% buffered neutral formalin, routinely processed, and embedded in paraffin wax. Sections were stained with Hematoxylin and Eosin (H & E). Final microscopic examination revealed a homogeneous population of large, round tumor cells with small nuclei and abundant eosinophilic granular cytoplasm infiltrating dense fibroconnective tissue (H & E, magnification ×40) (Fig. 3d). There were no signs of cell atypia, necrosis, or increased mitotic activity at higher magnification (H&E, magnification ×40) (Fig. 3d). On immunohistochemical staining, the granular cells were immunereactive for the proteins S-100 (Fig. 3e), SOX10, and calcitonin and also negative for desmin, myogenin, Smooth Muscle Antigen (SMA), Melan-A, and HMB-45. Proliferation activity of tumor cells for Ki67 was 1-2%.
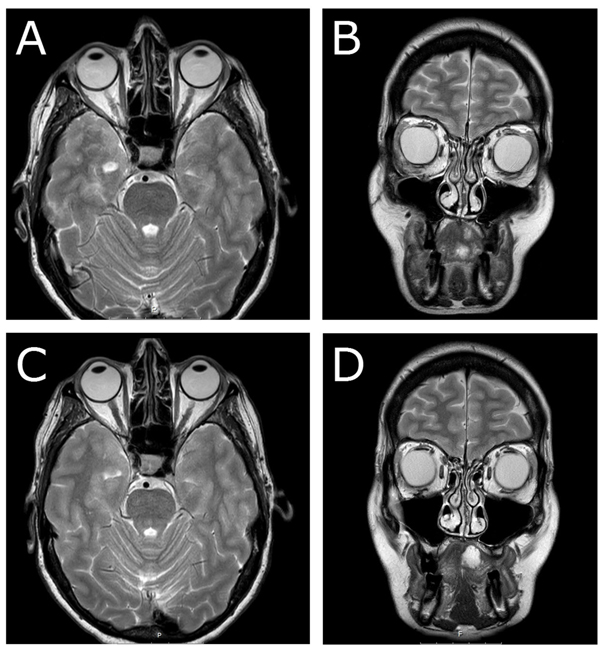
Postoperatively and until two months after surgery, the patient experienced diplopia in sight direction laterally downwards. The ophthalmological examination showed some reduction in elevation and lateral movement of the right eye. Six months later, MRI radiography revealed postoperative fibrosis with no signs of inferior rectus muscle entrapment (Figs. 4a & 4b). At 1.5-year follow-up, no diplopia was recorded. Ophthalmological examination revealed intraocular pressure of 15 mm Hg on the right side and 14 mm Hg on the left side, normal ocular motility, and visual acuity. In this case, no recurrence was noted at 1.5-year follow-up, either clinically or radiologically (Figs. 4c & 4d).
3. LITERATURE REVIEW
A PubMed search was performed up until April 2018 on the terms “Granular Cell Tumor” and “Orbit”. Case reports written in English were included. Cases written in other languages and those with ocular adnexa involvement were excluded.
Demographic data on age, gender, symptoms, duration of symptoms, ophthalmological findings and restriction in ocular movement were registered. We also analyzed treatment strategies and recurrence rate. The present paper is authored as a narrative review contribution.
3.1. Demographic Outcome and Clinical Manifestations
A PubMed search gave 35 articles in total. We excluded five articles (six cases) due to being written in German. Altogether, 30 articles (involving 38 cases, 15 males and 20 females with a mean age of 44.4 years (range 4-75 years) were included in the study. Demographic data are given in Table 1.
3.2. Treatment Strategies and Recurrence Rate
The different treatment strategies presented in previous studies included total resection, subtotal resection, biopsy and follow-up, and lastly additional treatment with radiotherapy and systemic chemotherapy. Recurrence rates for GCTs according to treatment, age, and site of origin are presented in Table 2.
| Authors and Years | No. of Cases | Age | Sex | Symptoms | Duration of Symptoms | Ophthalmological Findings | Restriction in Ocular Movement | Benign/Malignant |
|---|---|---|---|---|---|---|---|---|
| Abtahi et al. 2018* | 1 | 55 | F | Swelling | Not stated | Increased intraocular pressure | No | Benign |
| Yang et al. 2017 | 1 | 54 | M | Diplopia | Sudden onset | Ptosis | Lateral | Benign |
| Ullivieri et al.2017 | 1 | 36 | F | Diplopia, proptosis | 1 year | Ptosis | Not stated | Benign |
| Zhang et al. 2016 | 3 | 71 | F | Swelling | 1 month | Ptosis, lateral deviation of the globe | Not stated | Benign |
| 68 | F | Not stated | Not stated | Not stated | Not stated | Benign | ||
| 15 | M | Headache, pain | Sudden onset | Not stated | Not stated | Benign | ||
| Yuan et al. 2016 | 1 | 37 | F | Diplopia, photophobia | 6 months | Ptosis, abnormal light reflex, visual acuity affected | All directions | Benign |
| Morita et al. 2015 | 1 | 40 | F | Not stated | Not stated | Not stated | Not stated | Malignant |
| Germano et al. 2015 | 1 | 49 | F | Diplopia, proptosis, exophthalmos | 2 months | Ptosis, partial occlusion of the lid, visual acuity affected | Not stated | Benign |
| De La Vega et al. 2015 | 1 | 37 | M | Diplopia, headache | 2 months | Ptosis, lid retraction | Elevation and depression | Benign |
| Salour et al. 2013 | 1 | 50 | F | Diplopia, displacement of the eye globe | 4 years | Ptosis, visual acuity affected, afferent pupillary defect | Elevation and depression | Benign |
| Fernandes et al. 2012 | 1 | 53 | F | Proptosis | Slow progression | Upward displacement of the eye globe | Not stated | Benign |
| Ribeiro et al. 2012 | 1 | 74 | M | Diplopia, proptosis, ptosis | 3 years | Ptosis, superior displacement of the eye globe | Elevation, abduction, adduction | Benign |
| Guerriero et al. 2011 | 1 | 65 | F | Sudden blindness | 4 years | Ptosis, no light perception, forward displacement of the eye globe | All directions | Benign |
| Poyraz et al. 2009 | 1 | 53 | F | Diplopia, swelling | Long-standing | Superior displacement of the eye globe | Elevation | Benign |
| Golio et al. 2006 | 1 | 49 | F | Proptosis, displacement of the eye globe | 2 years | Visual acuity affected | Elevation | Benign |
| Ahdoot et al. 2005 | 1 | 56 | F | Asymptomatic | Not stated | Choroidal striae | Elevation | Benign |
| Callejo et al. 20001 | 1 | 72 | M | Diplopia, proptosis | Not stated | Ptosis, visual acuity affected | Abduction and adduction | Malignant |
| Allaire et al. 1995 | 1 | 35 | F | Diplopia, swelling | 2 months | Swelling of lower lid | Elevation and depression | Benign |
| Rodriguez et al. 1993 | 1 | 56 | M | Diplopia | 1 year | Limitation of downward vision, torticollis | Elevation and depression | Benign |
| McNab et al. 1991 | 4 | 4 | F |
Proptosis left divergent squint | 3 months | Ptosis, visual acuity affected, reduced color perception | Elevation and adduction | Benign |
| 27 | F | Ptosis | 1 year | Ptosis | No | Benign | ||
| 54 | M | Diplopia, displacement of the eye globe | 10 months | Hypertropia | Depression | Benign | ||
| 37 | M | Diplopia | 8 months | Ptosis, visual acuity affected, lateral displacement of the eye globe | Abduction | Benign | ||
| Moseley et al. 1991 | 4 | 36 | M | Diplopia, pain | 1 year | Ptosis, afferent pupillary defect and edema | No | Benign |
| 51 | M | Diplopia, swelling | 1 year | Palpable mass in lower lid, superior displacement of the eye globe | Not stated | Benign | ||
| 29 | F | Ptosis, lid lag | 1 year | Not stated | Not stated | Benign | ||
| 4 | F | Proptosis, left divergent squint | Rapid progression | Visual acuity affected | Not stated | Benign | ||
| Jaeger et al. 1987 | 1 | 71 | F | Swelling | 1 month | Not stated | Not stated | Benign |
| Dolman et al. 1987 | 1 | 44 | M | Diplopia | 11 months | Visual acuity affected, superior displacement of the eye globe | Elevation, abduction | Benign |
| Ueda et al. 1986 | 1 | 18 | F | Diplopia, pain, exophthalmos | 2 years | Ptosis, visual acuity affected | Not stated | Benign |
| Singleton et al. 1983 | 1 | 42 | F | Diplopia, displacement of the eye globe | 2 years | Not stated | Not stated | Benign |
| Karcioglu et al. 1983 | 1 | 65 | F | Proptosis, visual acuity affected | 2 years | Visual acuity affected, superior displacement of the eye globe | Downward and inferior | Benign |
| Goldstein BG et al. 1982 | 1 | 51 | F | Diplopia | 1 months | Visual acuity affected, superior displacement of the eye globe | Downward | Benign |
| Drummond et al. 1979 | 1 | 43 | F | Proptosis, visual acuity affected | 3 months | Visual acuity affected, anterior displacement of the eye globe | No | Benign |
| Müller et al. 1978 | 1 | 75 | M | Left-sided deafness, loss of sight | 6 years | Left-sided blindness, atrophy of optic nerve | Not stated | Malignant |
| Gonzalez et al. 1975 | 1 | 8 | M | Epiphora, conjunctival hyperemia, pain | 7 months | Visual acuity affected | Not stated | Benign |
| Shang-Hsien et al. 1955 | 1 | 23 | M | Diplopia, proptosis, headache | 2 months | Visual acuity affected, upper lid dropped | Downward, outward | Benign |
| Dunnington et al. 1948 | 2 | 36 | F | Proptosis, Drooping of the lid | 19 months | Ptosis | Upward | Malignant |
| 40 | M | Redness and swelling of the lid | Not stated | Upward and lateral displacement of the eye globe | Not stated | Benign |
| Variable | Recurrence No. (frequency) | No recurrence No. (frequency) | Unknown No. (frequency) | Total | Length of Follow-up |
|---|---|---|---|---|---|
|
Treatment Total resection Subtotal resection Other treatment* Biopsy and observation |
3 (10%) 3 (3.7%) 2 (100%) 0 (0%) |
19 (63.3%) 2 (2.5%) 0 (0%) No growth 1 (100%) |
8 (26.7%) 3 (3.7%) 0 (0%) 0 (0%) |
30 8 2 1 |
0-6 y 0-2 y 2 y 2 y |
|
Age, y < 35 ≥ 35 |
1/6 5/6 |
4/22 18/22 |
3/11 8/11 |
39 39 |
– |
|
Site of origin Inferior orbit Inferomedial orbit Inferolateral orbit Superior orbit Medial orbit Retrobulbar Orbital apex Inferior, medial, lateral orbit Superiolateral orbit Orbital apex Orbital fissure |
2 (18%) 1 (50%) 0 (0%) 1 (16.7%) 1 (25%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 1 (50%) – |
5 (45.5%) 1 (50%) 1 (33.3%) 4 (66.7%) 3 (75%) 3 (100%) 1 (100%) 1 (100%) 1 (100%) 1 (100%) 1 (100%) 0/2 |
7 (63.6%) 0 (0%) 2 (66.7%) 1 (16.7%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 1 (50%) – |
14 2 3 6 4 3 1 1 1 1 1 2 |
unknown-6 y unknown-1 y unknown-4y unknown-2y 6m-8y 2-3y 2y 6m 15m 2y 2y 2y 9m |
| Total | 6 | 22 | 11 | 39 | – |
4. DISCUSSION
We have presented a case of GCT within the right orbit in a 55-year-old woman, along with a review of 38 cases in the recent literature [4, 6, 14-37, 40]. In the case presented, the tumor was successfully resected with no recurrence or postoperative sequelae during the 18 months of follow-up. Physical examination revealed no other abnormalities or visual disturbances. Slit-lamp biomicroscopy showed a subconjunctival mass adjacent to the right inferior rectus muscle. In this case report, a pre-surgical biopsy was necessary to differentiate between a benign and a malignant lesion. In the literature, other lesions such as lymphoma, lacrimal cyst, meningioma, schwannoma, and glioma might be considered as differential diagnoses to GCT in this area [4, 5]. In the present study, the patient was referred with a 2-year history of swelling at the lateral border of the right eye without any other symptoms. Previous case reports and case series on GCT of the orbit showed that the most common symptoms were diplopia (55%) and proptosis (21%), the mean duration of symptoms being 15.6 months (range 1-72 months) (Table 1).
MRI is superior to CT-scan for evaluation of ocular muscles and soft tissue within the orbit in relation to the tumor mass [18]. The present case showed characteristic features of GCT on MRI: An oval lesion with circumscribed borders which was isointense to gray matter relative to extraocular muscles on T1-weighted images and hypointense on T2-weighted images relative to fat, and showed strong contrast enhancement. CT-scan and MRI reveal information such as tumor size, extent of the tumor, and metastatic spread. These modalities cannot differentiate between GCT and other benign or malignant tumors of the orbit.
Histological analysis of a tumor is regarded as the gold standard for distinguishing malignant tumors from benign. In the literature, malignant presentation accounts for approximately.
1-3% of all GCTs [18]. Fanburg-Smith et al., put forward six histological criteria for malignant diagnosis: (1) necrosis, (2) spindling, (3) vesicular nuclei with large nucleoli, (4) increased mitotic activity (> 2 mitoses per 10 high-power fields at 200× magnification), (5) high nuclear to cytoplasmic ratio, and (6) pleomorphism [12]. The presence of three or more of these criteria confirms the diagnosis of malignant lesion. In the case of pleomorphism only, without any of the other criteria, the tumor is classified as benign. We found a homogeneous population of large round tumor cells with small nuclei and abundant eosinophilic granular cytoplasm, infiltrating dense fibroconnective tissue. There were no signs of cell atypia, cell pleomorphism, necrosis, or increased mitotic activity. This is important to consider during the pre-surgical evaluation and highlights the importance of excisional biopsy in treatment strategies for GCT. In the present literature review, four of 39 GCTs in the orbit were malignant (10.5%), which is a considerably higher frequency of MGCT than previously reported from other sites of the body.
The immunohistochemical characteristics of GCTs have been discussed in the literature and their probable origin from Schwann cells has also been considered [8, 17, 38]. These tumors are positive for expression of protein S-100, which supports the neural origin of GCTs [19]. Furthermore, it has been found that the granular cells are also positive for inhibin-a (a peptide hormone that participates in the regulation of the pituitary gland) and CD68 (a macrophage marker) [39]. Expression of CD68 in GCTs is related to the intracytoplasmic accumulation of phagolysosomes.
Total surgical removal of the tumor with tumor-free margins is the optimum treatment option. This may be difficult to achieve in cases with extraocular muscle involvement. In the literature, the most affected extraocular muscles are the inferior rectus (26%) and medial rectus (38%) [4]. At the time of diagnosis, almost 80% of patients show diplopia, and after surgical resection, the diplopia persists in 73% of cases [4, 40]. Previous studies have shown a recurrence rate of 16% for all treatment approaches (Table 2). After total surgical resection and subtotal resection, the recurrence rate has been 10% and 37%, respectively [27].In the present case, MRI examination showed a solitary soft-tissue mass with involvement of the rectus inferior muscle. The surgical removal of the tumor in our case was challenging, due to the risk of permanent extraocular dysfunction. We removed the tumor with approximately 1 mm of the right lateral border of the inferior rectus muscle. This was necessary to avoid recurrence, and when there is diplopia various strabismus procedures would have to be used for primary ocular alignment.
MGCTs are rare, and only a few cases have been reported in the literature. Fanburg-Smith et al. presented 28 cases of MGCT and found local recurrences in 32% and metastases in 50% of the cases [12]. Eleven of the 28 patients with MGCT (39%) died of the disease after a median time interval of 3 years. Again, these findings highlight the importance of excisional biopsy for correct diagnosis and treatment strategies. In malignant cases, monotherapy with pazopanib (a tyrosine kinase inhibitor) in combination with radiotherapy and wide surgical excision of the tumor would be the treatment of choice [19]. However, more studies are needed to confirm these findings.
CONCLUSION
The occurrence of GCT within the orbit is a rare event and a PubMed search gave a total of 39 cases. GCT should be considered in the differential diagnosis of orbital tumors and a biopsy will often be required to exclude malignancy. The choice of treatment for GCTs is complete surgical resection, and the recurrence rate is low.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for her anonymized information to be published in this article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


