All published articles of this journal are available on ScienceDirect.
Modes of Action and Clinical Efficacy of Particulate Hydroxyapatite in Preventive Oral Health Care − State of the Art
Abstract
Background:
Particulate Hydroxyapatite (HAP; Ca5(PO4)3(OH)) is being increasingly used as multifunctional active ingredient in oral care. Due to its high similarity to human enamel crystallites, it is considered as a biomimetic agent.
Objective:
The aim of this narrative review is to identify the modes of action of HAP in preventive oral health care based on published studies. The outcomes are expected to improve the understanding of the effects of HAP in the oral cavity and to provide a knowledge base for future research in the field of biomimetic oral care.
Methods:
The data analyzed and discussed are primarily based on selected published scientific studies and reviews from in vivo, in situ, and in vitro studies on HAP in the field of preventive oral health care. The databases Cochrane Library, EBSCO, PubMed and SciFinder were used for literature search.
Results:
We identified different modes of action of HAP in the oral cavity. They are mainly based on (I) Physical principles (e.g. attachment of HAP-particles to the tooth surface and cleaning properties), (II) Bio-chemical principles (e.g. source of calcium and phosphate ions under acidic conditions and formation of an interface between HAP-particles and the enamel), and (III) Biological principles (e.g. HAP-particles interacting with microorganisms).
Conclusion:
Although more mechanistic studies are needed, published data show that HAP has multiple modes of action in the oral cavity. Since the effects address a wide range of oral health problems, HAP is a biomimetic agent with a broad range of applications in preventive oral health care.
1. BACKGROUND
1.1. Teeth
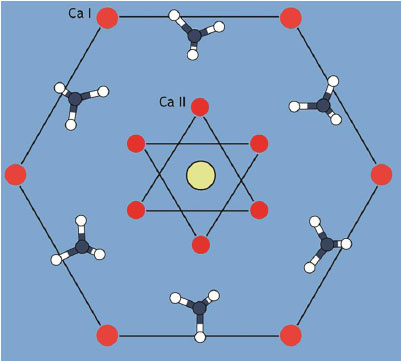
The mineral phase of human tooth enamel and dentin consists of Hydroxyapatite (HAP; Ca5(PO4)3(OH)), a crystal line calcium phosphate (Fig. 1). Enamel has an average HAP content of 97% while dentin is far less mineralized with an average HAP content of 70% [1-6]. From an evolutionary point of view, tooth enamel consisting of HAP is an ancestral character present in the large majority of vertebrate groups. The only notable exception are sharks and a small number of bony fishes whose teeth are covered with enamel-like enameloid consisting of fluoroapatite (Ca5(PO4)3F) [4, 7, 8]. Under healthy conditions, human tooth enamel is already fully mineralized when a tooth erupts [9]. The mineralization of enamel is a highly complex process, where ameloblasts, the enamel-building cells, interact with calcium and phosphate ions building the HAP-crystals and finally the enamel prisms [9-11]. Enamel is a tissue that cannot be rebuilt naturally [11]. Teeth are subjected to constant mechanical and chemical wear often accompanied by various clinical conditions during their service. Both phenomena are promoted to different extents by locally varying dietary habits [12, 13]. In consequence, almost the whole world population is affected by negative enamel and dentin conditions with the major factors being excessive erosion and caries [12, 14].
1.2. Hydroxyapatite in Oral Care
Contemporary everyday oral care strategies focus on the protection and preservation of enamel and dentin, mainly by using fluorides [15-17]. In the field of clinical dentistry, tooth repair and replacement are relying on ceramics, composites, polymers, and other materials [18, 19]. Despite a constant increase both in the treatment as well as financial efforts, the problems with dental health still prevail [20, 21]. Therefore, the demand for alternative and complementary treatment strategies is on the rise and researchers are increasingly investigating new approaches [3, 6, 22-30]. One strategy focuses on the analysis of bioinspired concepts that rely on mimicking the natural constituents of human teeth like crystalline calcium phosphate minerals [3, 6, 22-26, 31]. Among the group of these calcium phosphates, HAP is the most extensively investigated in the field of oral care e.g. [1, 3, 6, 26, 27, 32]. Dorozhkin & Epple [1] and Enax & Epple [3], for example, describe the synthesis routes and characterization of various non-biogenic apatites. In addition to synthetic HAP, HAP from natural sources (e.g. bovine bone as well as fish bone and scales) can be used for medical applications [33].
Several studies show the abilities of HAP to remineralize early caries lesions [3, 6, 24, 26, 34, 35]. The effectiveness of HAP as active ingredient is not limited by the quantity of calcium and phosphate present in saliva, since HAP can intrinsically act as source for these ions. This as well as its high chemical and structural similarity to natural enamel crystallites [1, 3, 24, 36, 37] qualifies HAP as a promising preventive oral health care agent.
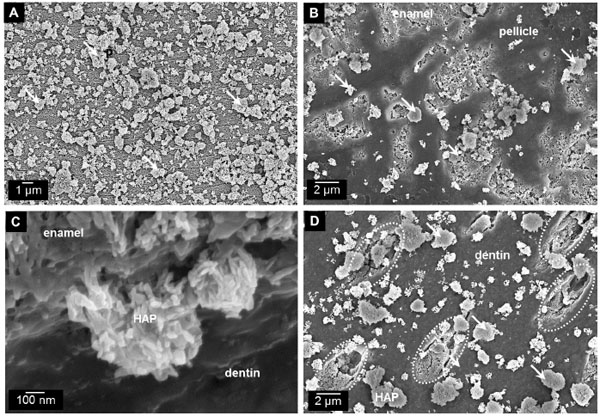
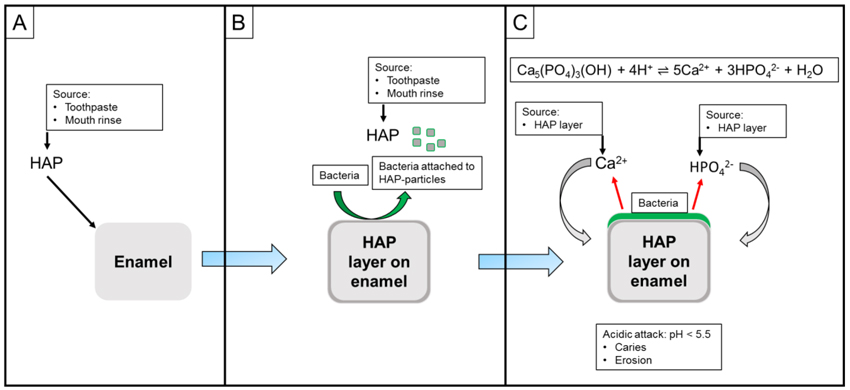
The similarity to human enamel and dentin crystallites is the reason why particulate HAP is already in use as a biomimetic agent in oral care formulations [3, 6, 22, 31, 32, 38, 39]. Several clinical trials have demonstrated the effectiveness of HAP-toothpastes regarding plaque reduction, improvement of periodontal health, and caries prophylaxis among caries-risk individuals [25, 26, 34, 40, 41]. Hu et al. recently showed in a meta-analysis the clinical evidence of HAP with reducing dentin hypersensitivity [27]. Compositional and structural accordance with tooth minerals confers an excellent biocompatibility to HAP [1, 24, 42, 43]. Consequently, the applicable dosage of HAP is not quantitatively restricted, and no fluorosis-risk is present [31, 44, 45]. Therefore, HAP as active ingredient may be especially suitable for individuals where high fluoride exposure is not recommended such as children (risk of swallowing), pregnant women, and individuals suf fering from hyposalivation (i.e. lack of calcium and phosphate ions for remineralization) [31, 35, 46]. Another important factor is the ability of HAP-particles to interact with enamel and dentin surfaces (Fig. 2) [23, 24, 47, 48].
Despite this wide range of applications in preventive oral health care, the exact modes of action of HAP in some areas remain quite elusive. References indicate that key effects are related to the ability of HAP to bio-chemically interact with tooth tissue as well as intrinsic and extrinsic substances present in the oral cavity, such as salivary components or microorganisms. Other effects seem to be more related to the particulate delivery form. In either case, understanding HAP’s mode of action is very important to exploit the full potential of HAP to improve its efficacy in biomimetic oral care formulations and to enable effective evidence-based oral hygiene recommendations for patients.
1.3. Aims and Literature Search Strategy
To date, a review that correlates the various described effects of HAP on different oral/tooth tissues (enamel, dentin, and gingiva) and oral diseases with the underlying modes of action has, to our knowledge, not been presented yet. Therefore, the aim of this narrative review is to analyze published scientific studies related to the effects of HAP in the clinical management of different oral and dental diseases. Based on the numerous studies recently reviewed by Meyer et al. and Enax & Epple [3, 6] as well as a further comprehensive literature search, the authors develop hypotheses about the modes of action of HAP used in oral care formulations with a focus on the prevention of caries, periodontitis, dental erosion, and dentin hypersensitivity. Cochrane Library, EBSCO, PubMed, and SciFinder were screened for relevant studies. Full text search terms were: hydroxyapatite AND (in vitro study OR in situ study OR in vivo study OR clinical study AND bacteria OR plaque OR biofilm OR caries OR periodontitis OR remineralization) AND (toothpaste OR dentifrice OR mouth rinse OR mouthwash) (Fig. 3).
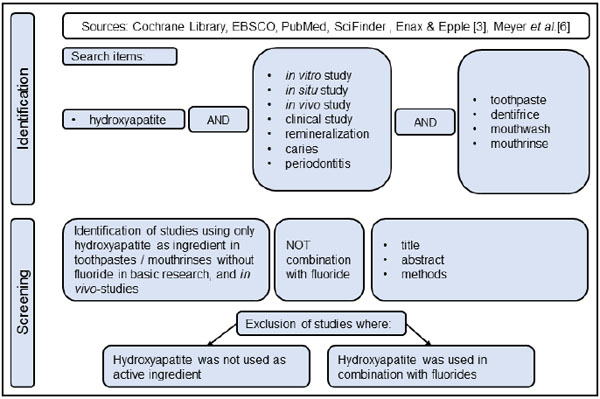
We correlate the objectives of the studies with proposed mechanisms of HAP in preventive oral health care (e.g. prevention of caries, periodontitis, dental erosion, dentin hypersensitivity) and conclude presumable modes of action based on physical, bio-chemical, and biological principles as well as combinations of these.
2. SHORT OVERVIEW OF PREVALENT ORAL DISEASES THAT ARE AFFECTED BY ORAL HYGIENE HABITS
The oral cavity is a highly complex environment that can be affected by a variety of diseases, some of which are highly prevalent among our society. The most predominant of which are dental caries, periodontitis, erosion, and dentin hypersensitivity. All of these have in common that they can be prevented up to a certain level without clinical intervention, namely by oral hygiene habits, reduced sugar (and acid) diet, smoking cessation, and stress-free life style.
2.1. Caries
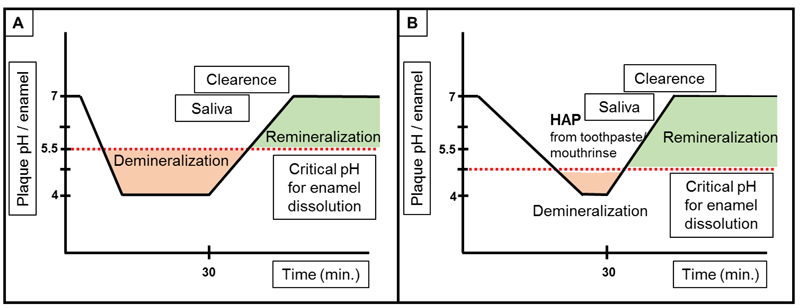
Caries is a disease that affects nearly every individual at least once in their life time and has the highest prevalence among all diseases that are known worldwide [49]. Even though its prevalence has declined in some parts of the world over the last years, it is still prevalent in all age groups and its treatment remains the highest unmet need in the world [20, 31, 50, 51]. Untreated caries affects more than 2.4 billion people around the world [20]. Caries is a biofilm-driven disease where the biofilm switches from homeostasis to dysbiosis [52]. Microorganisms that form biofilms on tooth surfaces metabolize sugars as energy-source, producing organic acids, mostly lactic acid, as end-products [53, 54]. By locally lowering the pH within the biofilm on the tooth surface, these acids are able to demineralize the underlying tooth tissue [1, 55]. This process is slowed down to individually different extents by the natural remineralization process, the rate and the extent of which depend on the availability of free calcium and phosphates from saliva and extrinsic sources. Local dissolution/recrystallization processes of the enamel surface on the microscopic scale caused by local concentration gradients may be an additional reason for superficial remineralization phenomena.
2.2. Periodontitis
Periodontitis is an inflammatory disease affecting the whole periodontium. In most cases periodontitis will start with gum inflammation and bleeding, also known as gingivitis [56]. Periodontitis can affect up to 90% of the population, while the severe form of this disease can be detected within up to 20% of some populations [50, 56]. Periodontal disease is one of the most prevalent diseases in the world (rank 11 in 2016) [21, 49, 57]. The incidence of periodontitis increases with the age, as teeth nowadays remain longer in the oral cavity [21]. Periodontal disease is caused by a dysbiotic state of the dental plaque and can be triggered by a suppressed immune response or genetic factors [58-60]. Traditional agents for prevention of this inflammatory disease focus on antimicrobial effects [61, 62]. Frequently used oral care products are, for example, based on chlorhexidine as well as stannous and zinc salts [30, 62, 63]. All of these antimicrobial agents might lead to a dysbiosis of the oral microbiota. In contrast to that, the modern approach in preventing periodontitis is focused on keeping the ecological balance of the microbiota: Not killing but controlling the harmful microorganisms [61, 64].
2.3. Erosion
Dental erosion, otherwise known as erosive tooth wear, is a dissolution primarily of the enamel caused by acids of non-bacterial origin, both from extrinsic or intrinsic sources such as seen with patients suffering from gastroesophageal disease or bulimia [14, 65-67]. Erosion is an increasing challenge for dentists, oral care practitioners and manufacturers of oral care products [14, 65]. The prevalence known from studies varies between 6 and 100% depending on the age group and the geographical region [65]. The highest prevalence was found with children aged 9 to 17 years [65]. Within adults, the prevalence ranges between 4 and 83% [65]. Erosive tooth wear is found to be mainly a consequence of modern dietary habits with fruits, juices, lemonades, and other acidic aliments [14].
2.4. Dentin Hypersensitivity
Patients often report dentin hypersensitivity to their dentists [38]. Prevalence ranges from 3 to 98% within several studies [68]. Depending on the age group, women are slightly more affected than men and most of the people at the age between 30 and 40 years suffer from dentin hypersensitivity [68]. Buccal surfaces are mostly affected. Reasons might be gingival recession or abrasion at the cemento-enamel junction exposing the dentin into the oral cavity [69]. Patients describe dentin hypersensitivity as a short, sharp pain [38]. The pain will occur when the nerves in the pulp are stimulated via the liquid-filled tubules in exposed dentin when triggered by stimuli, typically thermal, evaporative, tactile, osmotic or chemical that cannot be ascribed to another defect or disease [38]. In general, two different concepts can be described to treat dentin hypersensitivity with oral care agents: Desensitization of the pulp / nerve and occlusion of dentinal tubules [32, 38, 70]. Occlusion might last longer due to physical mechanisms, as ions for desensitization (i.e. potassium) are only momentary pain-relieving.
3. HAP IN ORAL CARE
Studies show the efficacy of particulate synthetic HAP in prevention of caries [26, 34], remineralization of early stages of dentin and enamel caries [35, 71, 72], prevention of periodontitis [23, 25, 47], reduction of gingival bleeding [25, 41], prevention of acid erosion [48, 73, 74], and reduction of dentin hypersensitivity [27, 75-78]. The fact that HAP is effective within multiple clinical indications in a number of different preventive oral care applications strongly indicates that this biomimetic calcium phosphate mineral has different relevant modes of action. These are either related to inherent physico-chemical properties of HAP or the chosen administration form. To develop formulations that are even more efficacious in combating caries and other oral diseases, the understanding of these modes of action is a very important key. Following this, the effects as demonstrated by the results of the scientific studies are discussed with respect to presumable modes of action of HAP.
3.1. Studies and Mechanisms of HAP in Preventing Caries
3.1.1. Studies
(1) The first clinical trial using HAP in the field of caries prophylaxis was published in 1989 [34]. Two different schools with at least 200 fourth-graders were included in the study. The study duration was three years. One group used a (fluoride-free) toothpaste containing 5% HAP, and the other group used a placebo-toothpaste without any active ingredients. Every year, the dmft/DMFT was acquired. While on the level of the primary dentition only minor effects could be observed, the caries reduction on the level of newly-erupted teeth was statistically significant in the HAP-group compared to placebo (mean caries inhibition: about 45%) [34].
(2) These above results were confirmed by a randomized clinical trial (non-inferiority-trial) from Schlagenhauf et al. [79]. This study was performed at five German university-hospitals using high-caries risk-patients undergoing orthodontic treatment. Since it is well known that these patients develop caries within four weeks [80, 81], the study was conducted over a period of six months and the 147 patients were randomly distributed using either a (fluoride-free) toothpaste containing 10% microcrystalline HAP, and a toothpaste containing amine fluoride/stannous(II)fluoride (1400 ppm fluoride). Caries incidence was measured using ICDAS-scores 1 and 2. Out of the 133 participants overall who finished the study per protocol, the HAP-group was not inferior to the fluoride-group [79].
(3) Lelli et al. used extracted teeth from an in vivo study (so called ex-in vivo study) to investigate whether a calcium-layer can be observed after using a HAP-toothpaste. Due to orthodontic or prosthetic reasons, teeth had to be extracted. Participants had either brushed their teeth with a fluoride-toothpaste or with a HAP-toothpaste for at least 8 weeks. The extracted teeth were analyzed using scanning electron microscopy (SEM) to visualize a possibly formed superficial layer and energy dispersive X-ray spectroscopy (EDX) to analyze its elemental composition. While in the fluoride-group no additional layer could be observed, the use of HAP-toothpaste leads to the formation of a calcium phosphate-containing layer on top of the natural enamel [48].
(4) Najibfard et al. conducted a double-blind in situ study with thirty participants using enamel slabs with and without artificial carious lesions [35]. Using a crossover-design (washout-phase in between the phases was seven days), participants brushed for at least 28 days with a fluoride-toothpaste (1100 ppm fluoride) or a HAP-toothpaste (5% HAP and 10% HAP, respectively). The slabs were analyzed using microradiography to quantify mineral loss and lesion depth. The results showed that HAP-toothpaste prevented the enamel from demineralization. Additionally, remineralization of enamel was not different between the toothpastes containing fluoride (1100 ppm fluoride), and HAP-toothpaste (5% and 10% HAP) [35].
(5) A clinical trial compared three different mouth rinses containing either HAP, CHX or fluoride with respect to plaque accumulation, gingival indices, and remineralization properties [41]. 81 children used the respective mouth rinse twice a day under parental supervision for two weeks. The children were examined after 1, 2, 4, and 6 weeks. With respect to caries (remineralization), HAP performed equally compared to fluoride and was also effective in reducing dental plaque and improving gingival health [41].
(6), (7) Two in situ studies investigated the bacterial adhesion to enamel surfaces after rinsing with an (6) aqueous dispersion containing HAP-particles [23] and (7) a mouth rinse containing HAP-particles [47]. Participants wore enamel slabs and rinsed with the respective dispersions. As positive control, CHX-containing mouth rinse was used, and negative control was also studied. Enamel slabs were carried in the oral cavity for six to twelve hours and then analyzed using bacterial staining and microscopy-techniques. Additionally, in vitro experiments regarding antimicrobial effects were also carried out. HAP does not kill the bacteria but leads to a decrease of bacterial attachment on enamel surfaces. The reduction of initial bacterial colonization to enamel surfaces in situ was comparable with the reduction of the antimicrobial agent chlorhexidine [23, 47].
Several in vitro studies have investigated the remineralization-potential of particulate HAP [71, 72, 82-84]. Most of the evaluated studies used demineralized enamel or dentin, and applied HAP-particles on these slabs [71, 83, 84]. Two studies, however, used pH-cycling models [72, 82]. Out of these, Esteves-Oliveira et al. did not detect a caries-preventive effect of HAP in vitro [82], while de Carvalho et al. described caries-inhibiting effects of HAP [72].
3.1.2. Mechanisms
The effects described in the results of the analyzed studies imply a number of different mechanisms where HAP plays a major role. As caries is a biofilm-associated disease, this biofilm should consequentially be reduced. HAP has already been shown to reduce the bacterial biofilm on the teeth [23]. Bacteria need a rough surface for attachment [85].
HAP in oral care products might help smoothen the surface through two different effects: Firstly, particulate HAP attaching to the enamel surface may preferentially deposit in small depressions such as scratches and superficial defects because it can more firmly attach and may be harder to remove by either abrasive food particles or brushing than on the smooth enamel surface areas, thus leveling the surface on the microscopic scale. Secondly, the remineralization effect and the resulting bio-chemically induced formation of a protective layer may also be more pronounced in surface depressions of the outer enamel. Both effects lead to a smoother surface that consequently decelerates the attachment of potentially harmful bacteria.
Furthermore, bacteria can erratically attach to individual HAP particles that are either loosely attached to the enamel surface or float in the oral cavity, thus inducing bacteria co-aggregation. In consequence, bacterial load will be reduced by spitting out the residual HAP after thorough tooth brushing. Despite all routine daily oral hygiene, bacteria can attach on the teeth after a certain time. If a protective layer of HAP particles is present and regularly sustained, this biofilm is formed on its surface instead of the calcium phosphate of the original enamel. When acids are produced within this biofilm, this protective layer might be dissolved, and calcium and phosphate ions will be released. This can have two main effects: On the one hand, the biofilm formation might be disrupted by influencing the energy metabolism of oral bacteria [86]. On the other hand, the excess of released calcium and phosphate ions become available for other, different effects. They might for instance help and promote remineralization of demineralized tooth surfaces and support the natural remineralization-mechanisms of saliva, i.e. shifting the equilibrium from de- to remineralization. If residual plaque is present (i.e. plaque that has not been removed by toothbrushing), HAP may also be incorporated into the biofilm as demonstrated for other calcium phosphates before [6, 87].
When acids are produced by bacteria, the dissociation products of HAP may act as buffering-solution. Particularly the phosphate ions are able to neutralize acids to a certain level, likewise the salivary phosphate-buffer. If present in excess, the released calcium and phosphate ions can also switch the solubility product balance back to remineralization.
In addition to the mechanisms described above, HAP can remineralize early caries-lesions directly which was shown under in situ conditions (Fig. 4) [35]. From a histological point of view, initial caries-lesions show larger distances between the rod-shaped HAP crystallites of the enamel than healthy portions. Small HAP particles from oral-care products might be able to penetrate through these gaps between enamel-rods and thus fill these initial lesions.
The HAP particles might bind to enamel/pellicle due to electrochemical forces, because of partial electronegativity: Zeta-potential of enamel and HAP-particles show a negative net surface potential of -15 to -30 mV, and basic proteins (i.e. from saliva/pellicle) a positive net surface potential of +20 to + 30 mV [88, 89]. HAP particles might also act as crystal nuclei by attracting calcium and phosphate from saliva [32]. Local concentration gradients can induce the formation of calcium phosphate through crystal growth on the enamel surface.
Since HAP is a calcium phosphate source itself, the described mechanisms do not only rely on ions provided with the salivary flow. As soon as bacteria are able to attach to a HAP-layer on tooth surfaces and acids are produced, calcium and phosphate ions will be released which may have a positive influence on the remineralization process (Fig. 5A-C).
HAP has a wide range of actions with respect to caries prevention. The main advantage of HAP compared to other oral care active ingredients is its high biocompatibility, i.e. no potentially toxically side effects like fluorosis. In consequence to this, there is no regulation in dosage [42, 43].
3.2. Studies and Mechanisms of HAP in Preventing Periodontitis
3.2.1. Studies
Several clinical trials report an improvement of gingival health after having used HAP-containing oral care products. (8) Harks et al. tested in a randomized clinical trial the differences in the plaque-formation-rate between a HAP-toothpaste and an antibacterial amine fluoride/stannous (II) fluoride-toothpaste. Sixty-seven patients with mild periodontitis completed the whole double-blind 12 weeks-trial. Besides plaque-formation-rate, several other clinical parameters were tested (i.e. bleeding on probing). The two groups used their respective toothpaste for 4 weeks without any therapy at home. After these 4 weeks supragingival cleaning was performed and the toothpastes were used for another 8 weeks. Plaque-formation rate did not change within that time. However, gingival health improved within both groups after 4 and 12 weeks. There were no differences between the antibacterial toothpaste and the HAP-toothpaste [25].
(9) Plaque samples from this study were also investigated using 16S rRNA amplicons for next generation sequencing. No differences of the plaque-composition could be observed between these two groups [90]. While mainly Fusobacterium and Prevotella species were observed in the interproximal and subgingival sites, buccal and lingual surfaces were dominated by Streptococcus and Veillonella species. However, the use of either antibacterial or anti-adhesive toothpastes did not change the composition noticeably indicating the stability of the ecological niche. Interestingly, gingival bleeding was reduced in both groups showing an effect on periodontal health of both treatments [25].
(10) Additionally, Cosola et al. tested a mouth rinse containing HAP (and zinc-PCA) with patients after surgical procedures [40]. Twenty-six patients were randomly allocated to either CHX-group or HAP-group. All patients received oral surgery. As postoperative treatment, patients rinsed with the mouth rinse. After their removal the suture threads from both groups, CHX and HAP (and zinc-PCA) were tested in vitro for colony-forming units. Despite different treatment, threads from both groups were found to have the same effect on bacterial growth. Since prevention of periodontitis relies on controlling bacterial growth, it is important to note that this study shows HAP (with addition of zinc-PCA) to reduce bacterial growth on suture threads. This goes in line with the in vivo (5) and in situ (6), (7) studies presented before [23, 25, 41].
3.2.2. Mechanisms
The results of these studies provide a number of useful hints with regard to HAP’s possible involvement in the prevention of periodontitis.
Dental plaque bacteria can interact with extrinsic HAP when present in the oral cavity. Thus, early colonizers, such as streptococci bind to free HAP derived from oral-care products instead of the tooth surface. The HAP can be either free particles in the oral cavity or particles bound to the tooth surfaces thereby creating a protective film of alternative substrate. As the early colonizers are needed for further biofilm-formation, the biofilm will be consequentially reduced either by directly spitting out of the free particles or by abrasion of the HAP layer through oral hygiene related cleaning activity.
It is known that gingivitis and periodontitis are promoted by a dysbiotic dental plaque if it is not removed. Hindering the settlement of early colonizers and reducing growth of the dental plaque can slow and potentially even prevent reaching a dysbiotic state.
The mode of action of HAP with respect to periodontitis is indirect. HAP is not able to directly interact with the immune system. Nevertheless, by reducing bacterial load and controlling the biofilm-formation, periodontal pathogens (i.e. Porphyromonas gingivalis and Tannerella forsythia) will not be able to lead to a shift in the taxonomic composition of the biofilm, as they are late colonizers and with that they are dependent of the early colonizers and bridge microorganisms (i.e. Fusobacterium nucleatum) [52, 62].
HAP also acts as an biomimetic cleaning agent (same hardness as the tooth) in toothpaste formulations, directly reducing dental plaque [32, 91].
The authors hypothesize that a HAP-layer applied to a tooth surface directly after mechanical dental plaque removal might lead to an aggravated biofilm formation [48]. Formulations with the addition of zinc salts or lactoferrin may boost the effect of HAP, especially against already existing or developing biofilms [30, 40, 92]. Zinc-substituted HAP, where Zn2+ions replace a part of the Ca2+ ions [5, 48], might also be dissolved in acidic environments, and the released zinc might act as an antimicrobial agent. This might promote the positive effects of HAP with respect to gingival health [25, 40, 47, 92].
In addition to that, HAP might also interact with proteins derived from microorganisms. Proteins are known to act as virulence factors, i.e. arginine deiminase arc A from P. gingivalis [59]. HAP particles present in the vicinity of the bacteria might bind these proteins [93]. Being immobilized, the virulence factors will not be able to interact with the human immune system.
Finally, HAP does not show unwanted side effects like discoloration of teeth or irritation of taste and can be used on a daily basis, in contrast to substances like chlorhexidine [23, 30, 47].
Furthermore, HAP does not kill bacteria (in contrast to e.g. stannous ions and chlorhexidine) but controls them by its physical presence or by passively withdrawing potentially harmful metabolic products from the oral cavity environment. Modern approaches focus on controlling the oral bacteria rather than killing them [94], since it is known that the use of antibacterial agents might lead to resistance or tolerance of bacteria against these agents. Biomimetic concepts have been shown to be promising alternatives or supplements in promoting periodontal health.
3.3. Studies and Mechanisms of HAP in Preventing Dental Erosion
3.3.1. Studies
Clinical studies in the field of erosion are rare due to a feasible study population and an acceptable study duration for the participants and ethical issues [95]. Therefore, in situ and in vitro studies using a sophisticated study design are much more important. (3) An ex-in vivo study by Lelli et al. has shown the formation of a (protective) calcium phosphate layer on top of the teeth in the HAP-group [48]. While some in vitro studies were not able to show a positive effect of HAP-toothpastes on erosion [96, 97], other studies were able to show protective effects of HAP-toothpastes [24, 71, 73, 74, 98, 99].
(11) Fabritius-Vilpoux et al. tested in vitro, if HAP-particles from an aqueous dispersion can attach to (mechanically and chemically eroded) enamel-surfaces [24]. Bovine enamel slabs were pre-treated with phosphoric acid. Afterwards, these slabs were dipped into agitated aqueous dispersions containing different HAP-concentrations. HAP particle attachment and surface-coverage was determined using SEM. Even a dispersion containing only 1% of HAP resulted in an area coverage of 10% of the eroded enamel surface. A dispersion with 10% HAP covered more than 30% of the enamel after one-time application [24].
(12) Another in vitro study used also enamel slabs and tested Vickers-hardness after erosive challenges and application of toothpastes [74]. Enamel slabs were erosively challenged four consecutive times (0, 8, 24, and 32 hrs.) for two minutes. After each challenge, toothpaste was applied on the surface. Vickers-hardness measurements revealed that acidic attacks significantly reduce enamel surface hardness. However, HAP-containing toothpastes caused significant re-hardening of the surface, indicating the occurrence of remineralization [74].
(13) The same group also used human enamel slabs in another study [99]. Here, the slabs were pre-treated with the respective toothpastes. Several fluoride-containing and calcium-containing toothpastes were tested besides HAP-toothpaste. These pre-treated slabs were then dipped into an acidic soft-drink for a maximum of 32 min. As measurement for erosive challenge, loss of weight of the slabs was determined. The HAP-toothpaste showed significantly less loss of mineral compared to the other products. This indicates a protective effect of HAP against erosive challenges [99].
(14) Colombo et al. used a visual rating system imaged by SEM to evaluate the enamel surface after application of a HAP-toothpaste [73]. Human incisor specimens were prepared and underwent an erosive challenge. In four different groups (positive control with no erosive treatment, negative control without toothpaste-application, fluoride toothpaste, HAP-toothpaste), different toothpastes were applied for 3 min. and rinsed off with distilled water at 0, 8, 24, and 36 hrs time intervals. In between the applications, specimens were stored in artificial saliva. Using a systematic assessment-method by SEM, enamel damage was recorded by four observers. The HAP-toothpaste was the only tested toothpaste where mineral deposition was observed after the erosive challenges. The grade of damage was consistently lowest in the HAP-group [73].
3.3.2. Mechanisms
From to above studies, one can deduce the following mechanisms: HAP forms, after application, a protective layer on the tooth surface. The protective layer might act as expendable shield that protects the tooth material from acidic attacks. Consequently, the protective HAP layer will be dissolved, releasing calcium- and phosphate ions that can also act as buffer system:
Ca5(PO4)3(OH) + 4 H+ → 5 Ca2+ + 3 HPO42- + H2O
Furthermore, it is known that the content of calcium within an erosive surrounding might lead to a shift of the equilibrium from dissolution to homeostasis. When parts of the teeth are eroded by acids, HAP is potentially able to remineralize attacked surfaces. Dental erosion and dental caries are both diseases caused by acids. However, the amount of acids and the origin of the acids is different. Consequently, pathogenesis of these two acid-driven diseases differs. Erosion is a fast process compared to caries: Acids produced by bacteria slowly penetrate through the outer layers of enamel to create a subsurface demineralization (caries), while extrinsic acids lead to a dissolution of the enamel’s outer layers (erosion). Enamel crystallites and prisms can be analyzed, when using SEM [73]. In erosion, HAP can cover the demineralized surfaces and might also directly remineralize the underlying demineralized tissue. In vitro experiments using clean enamel surfaces indicate that HAP particles attach to the surface of eroded teeth not only by electrochemical forces, but directly form solid interfaces between HAP crystallites from the particles and the enamel [24]. Hornby et al. performed several experiments where they used HAP in combination with fluoride treatment [100]. However, one experiment was performed using 45Ca-labelled HAP to determine Ca-uptake from a HAP slurry after an erosive attack. While the Ca2+-uptake from HAP with sound surfaces was 1.05 mg/mm2, Ca2+-uptake with demineralized enamel was three times higher (3.16 mg/mm2). The authors concluded that this indicates an availability of Ca2+-ions from HAP for remineralization [100].
In conclusion, HAP can form a protective layer on tooth surfaces and remineralize eroded enamel and dentin [48, 71, 73, 74, 99]. By forming a “sacrificial layer”, acidic attacks will not directly demineralize the teeth. Additionally, HAP leads to a shift of the solubility equilibrium (Fig. 6). Regular use of HAP is needed to cope with regular acidic challenges.
3.4. Studies and Mechanisms of HAP in Preventing Dentin Hypersensitivity
3.4.1. Studies
(15) Hu et al. conducted a systematic review and a meta-analysis that proved the effect of HAP in relieving dentin hypersensitivity [27]. The authors searched five databases for randomized controlled trials investigating dentin hypersensitivity pain relief. Risk of bias was assessed following the Cochrane guidelines. Confidence intervals and evidence were also calculated in this study. Particulate HAP was evidentially proven in relieving dentin hypersensitivity [27].
Amaechi et al. performed an (16)in situ study [28] and an (17) in vivo study [29] in the field of dentin hypersensitivity using HAP. (16) The in situ study tested the occlusion of dentin tubules after application of either 10% or 15% HAP [28]. Overall, 20 participants per group were recruited to wear human dentine blocks for at least 14 days. Additionally, a toothpaste containing fluoride and another containing NovaMin were also tested. Untreated blocks were used as control. After 7 and 14 days, respectively, dentine occlusion was visualized using SEM. Both tested concentrations showed after 7 and 14 days a higher degree (up to 50%) of completely occluded dentin tubules than the non-HAP toothpastes. In the 15% HAP-group, the test surfaces were 100% covered with a precipitate layer [28]. (17) These results were confirmed by the clinical trial from the same group where reduction of dentin hypersensitivity was investigated [29].
(18) Huettemann and Doenges published already in the year 1987 a double blind clinical trial using different toothpastes with HAP particles of different diameters [101]. 140 patients with dentine hypersensitivity were recruited. Study duration was four weeks and sensitivity was clinically tested using standardized tests (i.e. cold stimulus). The test pastes containing HAP (diameter 2 µm and 6 µm) were compared to a placebo. While in the placebo-group no improvement could be measured, 90% of the HAP-group reported an improvement of dentin hypersensitivity already after 3-5 days. 50% of the HAP-group were pain-free within the study-period (four weeks) [101]. These results were confirmed by several other clinical trials using HAP-containing formulations for relieving dentin hypersensitivity [75, 76, 78, 102].
(19) Hiller et al. tested the in vitro permeability of dentin after application of particulate HAP [77]. For this, they used bovine dentin slabs where a HAP-toothpaste was applied. Subsequently, the hydraulic conductance was tested and used as a measure for the degree of occlusion of the dentin tubules. Permeability was significantly reduced after application of all tested toothpastes, including the HAP-application [77].
3.4.2. Mechanisms
The mechanisms of HAP in preventing dentin hypersensitivity can be summarized as follows: In exposed dentin the dentinal tubules are open towards the oral cavity and to the pulp. Thus, external stimuli can propagate through the dentinal fluid directly to the nerve tissue in the pulp via the odontoblastic processes located in dentinal tubules. Since the applied HAP-particles have a high polarity, they are able to bind both to collagen and hydroxyapatite from dentin. Thus, these particles will attach to dentinal surfaces and eventually occlude exposed dentinal tubule openings if they are smaller than the tubule diameter. The size of tubules/diameter close to the tooth surface or the DEJ, respectively, is about 3.5 µm [103]. Most oral care products contain HAP-particles of sizes between 0.1 and 10 µm [38]. Particles need to have diameters <5 µm to reliably occlude dentinal tubules [38]. HAP particles close the tubules by being pressed into the tubule openings during for instance brushing. Within a certain time, HAP-particles that have occluded tubules will bio-chemically bind to the collagen-rich dentin and bio-chemically fuse with the inner mineral lining of the tubule. Deposited HAP soon gets mineralized by attracting calcium and phosphate ions from saliva. Mineralization will lead to fewer open tubules, and consequently less possibilities for external stimuli to induce pain. When using HAP containing oral care products regularly, dentinal tubules can be completely occluded due to the filling effect building up over time and the concurrent formation of a protective layer on the dentin. As positive side effect, dentin might also be protected from acidic attacks by the mineralized deposited HAP that can act as sacrificial layer.
In conclusion, HAP is known for decades to reduce dentin hypersensitivity. Several studies have shown a significant improvement of clinical parameters when using HAP-based oral care products, which is confirmed by a recently published meta-analysis [27].
3.5. Studies and Mechanisms of HAP in Promoting Tooth Whitening
3.5.1. Studies
Tooth whitening becomes more and more popular, because the social demand for whiter and brighter smiles is increasing [104]. Beside in-office bleaching, several oral care products for home use show whitening properties [105-108]. Most whitening toothpastes on the market are characterized by high abrasiveness. Their effect relies on removing the outermost stained layer of enamel which may lead to increased roughness or other side effects, such as dentin hypersensitivity [91, 104, 105, 109]. In addition to an increased amount of abrasives, toothpastes often use phosphate-systems for stain removal [106, 107]. However, only extrinsic stain can be removed when using whitening toothpastes. (20), (21), (22), (23), (24) There are studies showing an effect of HAP with respect to tooth whitening [110-114] and their results are promising. Basically, HAP acts as cleaning agent, but in contrast to other abrasives used for tooth whitening products (i.e. perlite and alumina) it has the same hardness as enamel [91]. Consequently, HAP does not lead to excessive enamel and dentin abrasion. (20) Dabanoglu et al. showed whitening properties of HAP. HAP-particles remained stable on the tooth surface after application of hydrodynamic shear force [110]. (21), (22), (23) The whitening-effect can be attributed to an attachment of HAP to the tooth surface, rather than a polishing process [111-113]. (23) The whitening effect of HAP could also originate from physical properties related to its particulate structure [112]. (24) In vivo results showed whitening effects of HAP-particles [114]. These results were verified with in vitro testing: Whitening properties of HAP are mainly based on diffuse reflection leading to optical whitening effects [114].
3.5.2. Mechanisms
Besides removing stain mechanically from tooth surfaces during routine oral hygiene, HAP particles might also bind proteins which often lead to discolorations. These would then be removed together with the particles during the cleaning process. The protective layer formed by HAP particles on tooth surfaces appears inherently white in colour as long as it remains firmly attached to the tooth surface. The size and irregular orientation of the HAP crystallites constituting the particles makes them ideal scatterers within the wavelength range of visible light. The resulting reflection of light makes the tooth surface appear even brighter white than natural, untreated tooth surfaces, especially if they are naturally darker tainted or contain incorporated amounts of extrinsic stains.
In conclusion, HAP particles are promising agents for tooth whitening, as they do not lead to tooth abrasion. HAP particles might remove staining proteins, cover tooth surfaces and enhance the perceived whiteness by scattering and reflection of light.
4. MODES OF ACTION OF HAP IN PREVENTIVE ORAL HEALTH CARE: CONCLUSIONS AND OUTLOOK
In contrast to other active ingredients in oral care, HAP represents a multifunctional biomimetic agent whose different effects in preventive oral health care are based both on its bio-chemical activity and the ability to physically interact with the oral cavity environment due to its particulate nature. The modes of action of HAP-particles in preventive oral care derived from data of the analyzed publications are summarized in Table 1. They are mainly based on the following principles:
| Effects | Modes of Action of HAP | Caries | Periodontitis | Erosion | Dentin Hypersensitivity | Whitening |
|---|---|---|---|---|---|---|
| Remineralization (dentin and enamel) |
Physical: Attachment and fusion of HAP-particles with tooth tissue: (3), (6), (7), (8), (11), (14) Bio-chemical: Local dissolution, ion donor, crystallization nucleus: (1), (2), (4), (5), (12), (13) |
X | --- | X | X | --- |
| Anti-adhesive effects (microorganisms, biomolecules) |
Physical: Loose/loosely attached HAP-particles as substrate substitute, ingestion and/or spit-out, abrasive effects during application: (6), (7), (8), (10) Bio-chemical: Binding affinity: (6), (7), (9) Biological: Settlement/substrate affinity of different microorganisms: (6), (7), (8) |
X | X | --- | --- | (X) |
| Protective layer |
Physical: Attachment of HAP-particles, layer formation: (3), (11), (21), (22), (23), (24) Bio-chemical: Formation of interphase at interface between HAP crystallites from tooth and synthetic (fusion): (11) |
X | X | X | X | X |
| Occlusion of dentin tubules |
Physical: Introduction of HAP-particles into tubules, layer formation: (15), (16), (17), (18), (19) Bio-chemical: Local dissolution, ion donor, crystallization nucleus, fusion: (19) |
--- | --- | --- | X | --- |
| Calcium and phosphate-reservoir |
Physical: Attachment of HAP-particles, layer formation: (3), (11), (14) Bio-chemical: Local dissolution through extrinsic causes, ion donor: (2) |
X | --- | X | --- | --- |
| Cleaning | Physical: HAP-particles as cleaning agent | X | X | --- | --- | X |
| Binding of proteins and carbohydrates |
Physical: Loose/loosely attached HAP-particles as substrate, ingestion and/or spit-out: (7) Bio-chemical: Binding affinity: (7) Biological: Settlement/substrate stimulus/repellent for different microorganisms: (7), (8), (9), (10) |
X | X | --- | --- | X |
| Compensatory substrate | Bio-chemical: Shift of the ion-balance to a supersaturated state of calcium and phosphate ions: (1), (2) | X | --- | X | X | --- |
(I) Physical principles (e.g. attachment of HAP-particles to the tooth surface and cleaning properties) [23, 24, 34, 48, 91].
(II) Bio-chemical principles (e.g. source of calcium and phosphate ions [100] and formation of an interface between HAP-particles and the enamel surface) [24].
(III) Biological principles (e.g. HAP-particles interact with microorganisms [reduction of bacterial colonization to tooth surfaces]) [23].
Several studies show the efficacy of HAP with respect to prevention and remineralization of caries, biofilm control, protection against erosion, and relief of dentin hypersensitivity [3, 6, 23, 25, 26, 35, 36, 41, 48, 71, 73-75, 77, 102]. Furthermore, HAP particles within oral care products can be used for tooth whitening [110, 114]. Due to its similarity to the tooth mineral phase, HAP is considered a biocompatible and biomimetic agent, and as such the applicable dosage is not limited by health concerns as it is the case with fluoride in fluorosis [42, 44]. Thus, oral care products with HAP can be used for all age-groups, including children. From the studies investigated and discussed it became evident that the effect of HAP on each of the clinical conditions relies on more than one mode of action. Therefore, HAP may experience future uses in other fields too. Based on the current state of the art, it can be concluded that HAP can be used as active biomimetic ingredient in preventive oral care for several indications. For future research, the physical, bio-chemical, and biological mechanisms of action need to be investigated in much greater detail using suitable in vitro and in vivo approaches, especially the interactions of HAP particles with teeth under conditions that mimic the situation in the oral cavity including pellicle, biofilms, and the entire spectrum of clinical conditions. This will enable to optimize the intrinsic modes of action and thus the efficiency of HAP by tailoring formulations of applications and the physical and chemical properties of the particles themselves.
In summary, HAP is a promising multifunctional biomimetic active ingredient with verified efficacy for different oral health concerns.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors thank Dr. Med. Dent. Barbara Simader for helpful discussions.


