All published articles of this journal are available on ScienceDirect.
In vitro Assessment of Peri-implantitis Treatment Procedures: A Review
Abstract
Background:
The prevalence of peri-implantitis is increasing continuously and such a biological complication significantly decreases implant survival and success. Although various treatment modalities have been identified for peri-implantitis, no completely efficient method has yet been established.
Objective:
The aim of this review was to evaluate the scientific literature regarding the in vitro effects of peri-implantitis treatment.
Methods:
A review of the literature was performed by using Google Scholar, PubMed/ MEDLINE and Science Direct databases. In vitro studies on peri-implantitis treatment modalities were selected. The search strategy identified 57 eligible studies. After selection, 21 articles met all the inclusion criteria and were included in the present review.
Results:
Included in vitro studies evaluated different types of peri-implantitis treatment modalities such as mechanical, chemical, combination and laser therapies. Combination therapies with the aid of adjuvants were found to be more effective compared to the studies that used only one type of treatment modality. Laser systems were also tested and displayed interesting results in terms of surface decontamination with a variability associated with selected parameters.
Conclusion:
This review was performed to evaluate the efficacy of the treatment modalities used for peri-implantitis in vitro. Although there are various effective treatment methods, none has been completely successful in removing the biofilms related to peri-implantitis. The findings imply the need for further studies to develop more effective antimicrobial treatment procedures.
1. INTRODUCTION
Nowadays, dental implants are considered as a key therapeutic tool to replace missing teeth. This treatment procedure allows the restoration of oral function and esthetics. Even if their long-term success is high, complications of mechanical but mostly of biological origin might occur [1]. Peri-implant diseases, such as mucositis and peri-implantitis, are considered leading causes of implant complications and implant loss [2]. Peri-implantitis is characterized by a progressive bone loss associated with inflammation induced by a microbial dysbiosis [3]. Indeed, this shift of composition within biofilms is concomitant with an increase in anaerobic bacterial species such as Porphyromonas gingivalis, Prevotella intermedia, Eikenella corrodens, Actinomyces naeslundi on the surface of the dental implants [1, 4]. The prevalence of peri-implantitis ranges from 4% to 45% according to the definition used and sample population [5].
Therefore, the elimination of dental plaque is the primary goal in peri-implantitis management and several treatment procedures have been suggested such as non-surgical approach with or without antimicrobials and surgical regenerative procedures [6]. However, mechanical curettage has been found to be insufficient for implant surface decontamination due to the presence of bacteria in surface micro-irregularities, and new therapeutic strategies have been developed to improve treatment outcomes [7]. For instance, laser treatments, antiseptics, and antibiotics have been reported as curative alternatives [8]. To develop and evaluate potential peri-implantitis treatment procedures, several in vitro studies have been and are still conducted. These studies evaluate the antibacterial, anti-inflammatory and also pro-regenerative effects of the tested procedures. This review aims to list the relevant in vitro models used and the outcomes of tested treatment procedures in the context of peri-implantitis management.
2. MATERIALS AND METHODS
2.1. Search Strategy, Eligibility, Inclusion and Exclusion Criteria
A review of the literature was performed using Google Scholar, PubMed/ MEDLINE and Science Direct databases, by searching for studies published after July 2012. Only in vitro studies assessing the treatment modalities of peri-implantitis were included. Additionally, a hand search was also carried out for cross-references of the selected articles. The keywords “peri-implantitis”, “treatment”, “in vitro”, “dental implant” were used on all databases in various combinations and alongside ‘and’, or, ‘not’.
The screening was performed by assessing the titles and abstracts of the research articles for initial analysis. Full-text of all relevant papers were obtained. The present review included papers based on the following inclusion criteria: 1)In-vitro studies including the types of treatments of peri-implantitis, 2) Studies including peri-implantitis and its microbiota, 3) Studies involving effective modalities or equipments as a treatment option for peri-implantitis, 4) Studies in English language, 5) Studies published in scientific journals. The exclusion criteria for this paper were as follows: 1)In- vivo studies, 2) Studies that did not present a compatible methodology, 3) Inadequate description of treatment modality for peri-implantitis, 4) Reviews and duplicates, 5) Studies with a lack of data regarding the extent of treatment modality or the method employed.
3. RESULTS
3.1. Study Selection
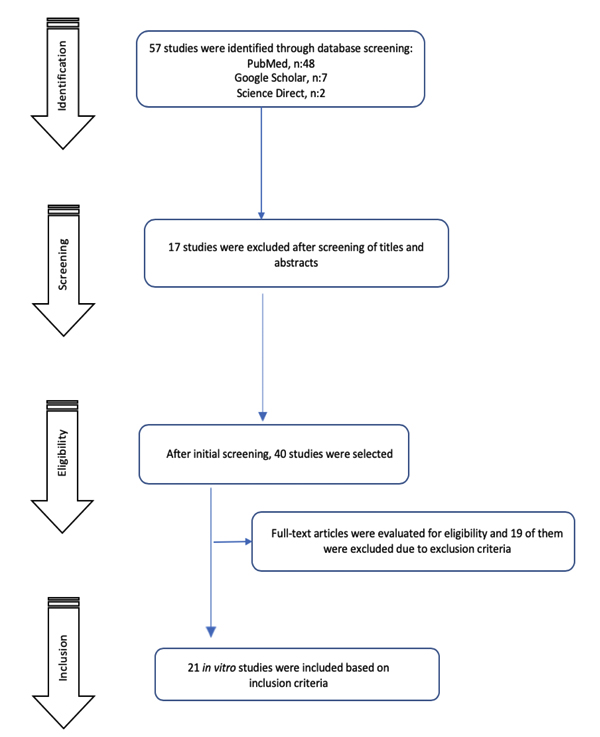
The search strategy identified 57 studies. After the screening procedure by reading abstracts and titles, 40 studies were found to be potentially eligible for this review. After full-text evaluation, 21 studies met all the selected criteria and were included. The flow diagram of article screening and selection process is shown in Fig. (1).
3.2. Study Characteristics
Among the selected in vitro studies, the authors evaluated the peri-implantitis treatment modalities using mainly titanium discs, dental implants and plan titanium specimens to mimic contaminated surfaces by different types of biofilms or planktonic bacteria including the main periodontal pathogens such as P.gingivalis, Treponema denticola, Aggregatibacter actinomycetemcomitans, Streptococcus oralis, Actinomyces viscosus, Veilloneilla parvula, Fusobacterium nucleatum and other species such as Escherichia coli, Staphyloccus epidermidis, Staphyloccus aureus or Candida albicans (Table 1).
3.3. Treatment Modalities
Several treatment modalities have been evaluated in vitro. These treatment modalities include mechanical procedures, chemical treatment through the use of antimicrobials and combinations (Table 1). Ultrasonic devices, manual curettes, air powder abrasion, titanium brush, implantoplasty, cold atmospheric pressure air plasma jet, electrolysis and laser have been assessed [9-12]. Regarding laser systems, diode, carbon dioxide, Er:YAG and GaAlAs lasers with different wavelengths, irradiation times and irradiation modes (pulsed and continuous) were tested [13-17]. Photodynamic therapy (PDT) through the use of laser and LED has been a popular treatment modality and was carried out for the decontamination of dental implant surfaces [15, 18-22].
Studies assessing antimicrobial treatments used different kinds of antiseptics and antimicrobial agents [19, 23, 24]. These topical antiseptics used to treat the surfaces of the specimens were chlorhexidine gluconate 0.2%, hydrogen peroxide 3.0%, sodium hypochlorite 1.0%, cetylpyridinium chloride, essential oils or citric acid 40.0% [19, 23]. Metronidazole, amoxicillin and the combination of these two antibiotics were used to treat titanium discs covered with bacterial biofilms [24]. Additionally, some other molecules or compounds were tested such as grape seed extract, oligosaccharide nanomedicine OligoG and Triethoxysilylpropyl Succinic Anhydride (TESPSA) silane application to determine their antimicrobial properties [25-27].
3.4. Synthesis of the Results
In the study performed by Bürgers et al., six different topical antiseptics were tested where only sodium hypochlorite 1.0% exhibited a significant antimicrobial effect on all the tested microbes [23]. In another study, hydrogen peroxide 3.0% and chlorhexidine 0.2% were tested and both were recommended for their antiseptic effect on three peri-implantitis associated microbiotas (S. aureus, S. epidermidis and C. albicans) [19].
Some natural compounds have also been evaluated. For instance, grape seed extract was tested firstly against peri-implantitis microflora. Interestingly, this extract inhibited S. aureus growth. However, modest or no effect against strains such as E. coli, C. albicans, C. parapsilosis, K. pneumonia was observed [25]. Another interesting study examined the potential role of oligosaccharide nanomedicine (OligoG), an antibiofilm adjuvant, and concluded that this agent was safe and non-toxic for clinical use and can help in preventing infection and attachment of P. gingivalis on implant surfaces [26]. The efficacy of the combination of amoxicilline/ metronidazole has also been advocated [24, 28].
| Authors | n | Groups | Sample Material | Bacteria | Intervention | Main Conclusion |
|---|---|---|---|---|---|---|
| Shrestha et al., (2012) | Not reported | 6 | Craniofacial implants |
S. aureus E. coli C. albicans K. pneumonia C.parapsilosis |
The use of grape seed extract for antimicrobial effects | Grape seed extract showed positive inhibitory effects only with S. aureus. Minimal or no reactivity against strains of other groups was detected. |
| Sahrmann et al., (2012) | 10 | 3 | Titanium discs |
S. oralis S. anginosus A. oris F. nucleatum V. dispar C. rectus P. intermedia P. gingivalis |
Treatment in an electrolytical setup with physiological saline and gelatin. | Electrolysis could be an effective means to disinfect implant surfaces. |
| Bürgers et al., (2012) | 5 | 7 | Plan titan specimens |
S. sanguinis S. epidermidis C. albicans |
Applying six different topical antiseptics on the surface of specimens. | Sodium hypochlorite was found to be effective against all the selected specimens. |
| Leja et al., (2012) | 4 | 4 | Dental implants | Not reported | Irradiation with diode, carbon dioxide, and Er:YAG lasers | Pulsed mode diode laser was recommended for preventing surface temperature. |
| Astasov-Frauenhoffer et al.,(2013) | Not reported | 3 | Titanium discs |
S. sanguinis F. nucleatum P. gingivalis |
Adjuvant antibiotic therapy of amoxicillin, metronidazole and their combination. | The combination of antibiotics was found more efficient than metronidazole alone on oral biofilms. |
| Roberts et al (2013) | Not reported | 3 | Titanium discs PMMA discs |
S. mutans P.gingivalis |
Applying oligosaccharide nanomedicine (OligoG) on Ti and PMMA surfaces. | The combination of OligoG and triclosan showed enhanced antimicrobial effect on oral biofilm. |
| Sahrmann et al., (2013) | 20 | 3 | Dental implants | Not reported | Applying curette, ultrasonic device and air powder abrasive for implant surface decontamination. | Airflow devices was shown to provide an efficient therapeutic effect for the debridement of implants in peri-implantitis defects. |
| Toma et al., (2015) | 10 | 4 | Titanium discs | Not reported | Treatment with plastic curette, air-abrasive device, titanium brush, and implantoplasty. | All groups appeared to be valid in terms of biocompatibility. |
| Widodo et al., (2016) | 19 | 6 | Titanium discs | S. aureus | Disinfection treatment with the use of six different modalities. | Combination of photodynamic therapy and Titanium brush modality was effective in reducing the number of selected bacteria. |
| Giannelli et al., (2016) | 9 | 3 | Titanium discs |
S. aureus E. coli |
Irradiation with diode laser of implant surfaces both pulsed and continuous modes. | The λ 808-nm diode laser appeared to be an efficient way for decontamination of titanium implant surfaces. |
| Valente et al., (2017) | 22 | 6 | Dental implants | S. sanguinis | Irradiation with two different diode lasers with/without the aid of photodynamic therapy. | Diode lasers were shown to be useful regardless of the aid of photodynamic therapy. |
| Rismanchian et al., (2017) | 5 | 18 | Titanium discs |
S. aureus S. epidermidis C. albicans |
Disinfection treatment with the use of five different modalities. | Combination of photodynamic therapy, H2O2 or 0.2% chlorhexidine, was recommended for disinfection of dental implant surfaces. |
| Giannelli et al., (2017) | Not reported | 4 | Titanium discs |
S. aureus E. coli |
Irradiation of implant surfaces with diode laser and LED in the concept of phototherapy. | Non-invasive phototherapy with LED appeared to an efficient method to reduce LPS and bacteria on implant surfaces. |
| Chellini et al., (2017) | Not reported | 3 | Titanium discs | Not reported | Irradiation with diode GaAlAs laser of implant surfaces both pulsed and continuous modes. | 808 ± 10 nm GaAlAs diode laser was shown to be an efficient treatment modality for peri-implantitis treatment. |
| Kuo et al., (2017) | Not reported | 3 | Dental implants | E. coli | Irradiation with Er: YAG laser of implant surfaces on pulsed mode. | Er: YAG laser treatment presented 98.9% sterilization rate on implant surfaces. |
| Vilarrasa et al., (2018) | 5 | 6 | Titanium discs |
S. oralis P. gingivalis A. viscosus V. parvula F. nucleatum |
Applying triethoxysilylpropyl succinic anhydride (TESPSA) silane (antibacterial surface treatment) on discs. | TESPSA was reported to reduce cellular viability and biofilm adhesion. |
| Yang et al., (2018) | 9 | 4 | Titanium discs | P. gingivalis | Treatment of cold atmospheric pressure air plasma jet was applied on discs. | Atmospheric pressure air plasma jet treatment appeared to be efficient in sterilization and bone formation. |
| Rogers et al., (2018) | Not reported | 2 | Bacterial biofilms |
P. gingivalis T. denticola |
Photodynamic therapy was carried out using a diode laser at 664nm. | Photodynamic therapy is an effective modality to eliminate microorganisms in peri-implantitis. |
| Monzavi et al., (2018) | 12 | 5 | Dental implants | Not reported | Surface decontamination was performed with five different laser types. | Er: YAG laser with the aid of photodynamic therapy was an efficient combination on implant decontamination. |
| Ghasemi et al., (2019) | 6 | 6 | Titanium discs | A. actinomycetemcomitans | Photodynamic therapy was carried out using a diode laser, LED and toluidine blue. | Photodynamic therapy using LED and toluidine blue was found more effective in the suppression of selected bacteria. |
| Huang et al., (2019) | Not reported | 9 | Titanium alloy plates |
S. mutans P. gingivalis A. actinomycetemcomitans |
Photodynamic therapy was carried out with different irradiation time, pH and methylene blue (MB) concentrations on Ti surfaces. | Photodynamic therapy with 200μg/mL MB at pH 10 for 60s of irradiation time appeared to be an efficient modality to eliminate LPS and bacteria. |
The study by Toma et al. evaluated four types of peri-implantitis treatment modalities: titanium brush (Ti-Brush), air-abrasive device (Perio-Flow), implantoplasty and plastic curette in terms of osteogenic effect and biocompatibility on titanium discs [11]. The authors found that although implantoplasty was the only treatment able to change the morphology of titanium surfaces, all modalities assessed in the study appeared to be valid in terms of biocompatibility and promoted mature osteoblastic phenotype as well as cell adhesion and proliferation. Sahrmann et al. evaluated the potential effect of decontamination of three different mechanical debridement modalities (ultrasonic device, air powder abrasive device and Gracey curette) and showed that airflow device was an efficient modality in peri-implantitis treatment with variable results according to the size of the defect [10]. Irradiation therapies with the use of laser systems are popular due to their decontamination efficiency on dental implant surfaces and in the treatment of peri-implant defects [29]. Included studies showed high rates of sterilization for laser systems on implant surfaces [16, 22, 30]. Additionally, changes in wavelengths and irradiation modes have been reported to influence the effectiveness of decontamination procedures [13, 14, 30].
Photodynamic therapy is another frequently suggested peri-implantitis treatment modality. PDT has been used alone and in combination with other treatment options [15-19, 21, 22]. In the study conducted by Widodo et al., the authors reported that the combination of PDT and titanium brush treatment was effective in reducing the number of selected bacteria [18]. Rismanchian et al. showed that PDT provided better treatment results when it was applied with 0.2% chlorhexidine or hydrogen peroxide 3.0%, and they recommended this modality for the disinfection of dental implant surfaces [19]. Ghasemi et al. proposed another combination of PDT with LED and toluidine blue, while Huang et al. highlighted the use of methylene blue. According to the results of these recent studies, both PDT protocols were found to be efficient for the decontamination of implant surfaces [16, 17].
4. DISCUSSION
The present review evaluated the treatment modalities for peri-implantitis and focused on their in vitro properties especially their antibacterial properties. Indeed, several modalities were proposed and tested with interesting outcomes and potential application in the management of peri-implantitis. However, significant differences in terms of the results were found according to the sample material, working mode, application time, type of system or combined usage.
An effective peri-implantitis treatment requires the following conditions: (1) Removing biofilm from the implant surfaces and (2) Promoting the adhesion of osteoblasts [31]. However, effective debridement of oral biofilms on dental implant surfaces is difficult to achieve due to the rough surface and the design of dental implants in contrast to the surfaces of natural teeth [32]. Therefore, several approaches have been developed to treat peri-implantitis [33, 34]. In some studies, contradictory results have been found. For instance, studies comparing several treatment procedures did not have the same conclusion. The differences between them may be due to the equipment used or sample materials.
Mechanical therapy induces significant changes in the composition and microstructure of titanium and implant surfaces as they might be damaged during treatment [11]. To reduce surface damage originated from metal-to-metal contact, a non-metallic instrument could be used [35]. However, these instruments have been shown to inadequately remove microorganisms from rough surfaces, and air-powder abrasive systems might be an interesting option. Implantoplasty also appeared to be a promising treatment because of inducing surface smoothness, surface hydrophily and purity [11]. Special attention should be paid to mechanical therapy because of the difficulty in visualizing the surface and to eliminate microorganisms [36]. Therefore, antibiotics or antiseptic agents have also been proposed to improve peri-implantitis treatment outcomes [15, 19, 23, 37].
Several classical antiseptic agents have been proposed for the management of peri-implantitis and peri-implant mucositis. Sodium hypochlorite, a strong disinfecting agent, has been tested but was not highly recommended due to its cytotoxicity [19, 23]. Common concentrations of hydrogen peroxide and non-toxic chemicals, provide rapid bactericidal effects and antimicrobial action [38, 39]. Chlorhexidine gluconate was assessed in many studies and showed broad antimicrobial activity by changing the bacterial membrane and resulting in cell destruction [19, 23, 39]. Citric acid has also been utilized as an antibacterial agent in detoxification of plaque infected implant surfaces and its efficacy has been proved [40, 41]. Essential oil (Listerine) also displayed antibacterial activity with enzymatic inhibition and cell wall destruction of microorganisms [42]. Triclosan served as an inhibitor of oral biofilm and is an effective antimicrobial product [43].
GaAlAs diode, Er:YAG, diode and carbon dioxide lasers have been used to treat peri-implantitis [13-16, 21, 30]. According to study protocols, treatment outcomes vary between studies. For instance, Kuo et al. recommended the use of Er:YAG lasers in pulsed mode for peri-implantitis treatment [30]. In the study performed by Leja et al., diode lasers in pulsed mode were found to be efficient, while Giannelli et al. reported continuous mode of diode lasers as more efficient than pulsed mode [13, 14]. The main risk of laser treatment is the extra heat generation in bone structures [44]. Contrary to this, laser treatment outcomes may change with the use of combination therapies such as PDT. The study conducted by Valente et al. showed that diode lasers have been useful regardless of the aid of photosenzitation, while others reported Er:YAG and diode lasers as effective treatment modalities with photosenzitation and highlighted efficient combination on implant decontamination [15, 17, 21, 22]. PDT has also been carried out in conjunction with different mechanical treatment, chemical agents, LED and laser systems. Only in the study performed by Valente et al., PDT was found to give a modest improvement regarding the surface decontamination and it was not statistically significant when compared to the group of diode laser treatment [15]. Other studies in the literature reported advanced treatment efficacy for PDT when it is performed as a combination therapy [15-19, 21, 22].
CONCLUSION
The present review was conducted to evaluate the in vitro effects of commonly used peri-implantitis treatment modalities. Although there is a wide range of treatment options, none of these modalities were able to completely remove peri-implantitis related biofilms in any of the affected surfaces. Further studies are needed to identify a capable and suitable treatment or combined modality treatment for peri-implantitis therapy. The impact of each treatment modality should also be take into account as, even minimal, surface modifications will increase the risk of bacterial recontamination.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


