All published articles of this journal are available on ScienceDirect.
Anxiety and Salivary Level of Alpha-Amylase in Patients with Geographic Tongue: A Case Control Study
Abstract
Background:
Geographic tongue is a common benign condition involving the tongue with an unknown etiology.
Objective:
This study aimed to measure the salivary level of alpha-amylase as well as the level of anxiety of patients with geographic tongue.
Methods:
This case-control study was performed on 180 subjects including 89 patients with geographic tongue and 91 controls. The subjects were requested to fill out the Spielberger’s State-Trait Anxiety Inventory (STAI-S, STAI-T). Unstimulated saliva samples were collected by the spitting method to assess the salivary level of alpha-amylase. Data were analyzed using SPSS version 23, t-test and Pearson’s test (α=0.05).
Results:
The mean salivary level of alpha-amylase and the mean scores of state and trait anxiety in geographic tongue patients were higher than those of healthy controls. But these differences did not reach statistical significance (P>0.05).
Conclusion:
Anxiety may be an influential factor in the occurrence of geographic tongue. Salivary level of alpha-amylase cannot serve as a specific biomarker for assessment of geographic tongue.
1. INTRODUCTION
Geographic tongue or erythema migrans is a common benign condition mainly involving the tongue. It is commonly found in routine examinations of the oral mucosa. It often affects the anterior two-thirds of the dorsal surface of the tongue. Multiple lesions appear with distinct margins and erythema, which is due to the atrophy of the filiform papillae of the tongue. Atrophic areas, at least in some parts, are surrounded by a slightly prominent, white to yellow, spiral or scalloped margins [1]. The white margin is comprised of the regenerating filiform papillae and a combination of keratin and neutrophils [2]. Lesions constantly migrate and undergo alterations in their shape and size. This migration is associated with epithelial loss in one region and stimulation of pro-liferation in another region and has periods of exacerbation and recovery [3]. Some patients complain of symptoms that appear following eating. Some others are concerned about the aesthetic appearance of their tongue and more importantly, there are patients worrying about the malignant transformation of the lesions and development of tongue cancer [4].
The etiology of this condition is still unknown [5]. Some studies have reported correlations between the occurrence of geographic tongue and diabetes mellitus [6], Reiter’s syndrome, Down syndrome [7], pregnancy [8], psychological factors [9, 10], family history [11] and intake of some medi-cations such as oral contraceptives [12] and lithium carbonate [13]. Moreover, a strong relationship has been reported between the geographic tongue and psoriasis [14]. Allergy is also considered as an important etiologic factor in the oc-currence of geographic tongue. Furthermore, geographic tongue is believed to be correlated with asthma, eczema, Hayfever, increased level of serum immunoglobulins and atopy [15].
Alpha-amylase is an important salivary enzyme [16]. It is the most abundant saliva protein and its endoglycosidase activity is the most commonly recognized activity of alpha-amylase. It also plays a role in the formation of pellicle on the tooth surface [17]. Amylase is secreted by the acinar serous cells of the parotid and submandibular glands; however, the parotid gland has a more prominent role in its production [18]. A recent study pointed to an association between secretion of alpha-amylase and high-stress conditions, and its increased concentration has been reported in both physical and mental stresses [19].
Nosratzehi et al. [20], evaluated the level of cortisol and alpha-amylase in the saliva of patients with burning mouth syndrome and showed a significant correlation between the salivary level of alpha-amylase and burning mouth syndrome. Cardoso et al. [21], evaluated the salivary level of alpha-amylase in patients with recurrent aphthous ulcers and demonstrated high variability in the salivary level of alpha-amylase in the two groups; however, they did not find a significant difference in this respect between the patient and control groups.
Since no previous study has assessed the salivary level of alpha-amylase in patients with geographic tongue, this study aimed to assess the salivary level of alpha-amylase as well as the level of anxiety in geographic tongue patients using Spielberger’s State-Trait Anxiety Inventory (STAI-S, STAI-T).
2. MATERIALS AND METHODS
This cross-sectional case-control study was conducted in the Oral Medicine Department of School of Dentistry, Isfahan University of Medical Sciences. Participants were selected among those presenting to this department for routine dental examinations from September to November 2017. The study was approved in the medical ethics committee of Isfahan University of Medical Sciences (code: 30406). Written informed consent was obtained from all participants prior to the study.
This study was conducted on 180 subjects in two groups of patient and control. The patient group included 89 patients with geographic tongue. Since the diagnosis of geographic tongue is based on clinical examination, patients suspected for this condition were examined by an oral medicine specialist to confirm the diagnosis of geographic tongue. The following criteria were used for clinical confirmation of geographic tongue diagnosis: (I) local absence of filiform papillae of the tongue, (II) irregular shape of the affected site, and (III) constant migration of the lesion. The patient group was categorized into three subgroups based on the number of lesions: mild (one lesion), moderate (2-5 lesions) and severe (6 or more lesions). The control group included 91 healthy participants who matched the patient group in terms of age, gender, marital status, level of education and socioeconomic status. Patients with a history of taking systemic medications especially beta-blockers, tetracycline, enalapril or captopril, those taking contraceptives in the past 2 weeks, history of steroid therapy in the past 3 months, affliction with any systemic disease specially immunologic or dermatologic conditions such as lichen planus, lupus erythematosus, pso-riasis and asthma, affliction with an oral inflammatory con-dition such as chronic or aggressive periodontitis and those with a history of salivary gland diseases were all excluded.
All participants were over 18 years of age, non-smoker and non-alcoholic. Women were not pregnant and all participants were mentally healthy and could fill out the questionnaire.
Unstimulated saliva was collected using the spitting method. All saliva samples were collected between 8-11 a.m. while subjects were fasting for 1-2 hours. They were asked to comfortably sit on a chair, rinse their mouth and after 5 minutes, spit into a sterile glass test tube every 60 seconds for a total duration of 5 minutes. The saliva samples were sent to a laboratory in a cooler container and stored at -20°C. Alpha-amylase activity kit (ZellBio GmbH, Ulm, Germany) was used for measurement of the salivary level of alpha-amylase. This kit measures the activity of alpha-amylase by colorimetry such that the sample is incubated with a starch-containing substrate and the reduction in blue color after the addition of iodine is compared with that in the control group. The absorbance range of alpha-amylase is 578-660 nm.
Level of the anxiety of participants was evaluated using the Spielberger’s STAI, which assesses both the state anxiety (the patient’s response to a specific stressful situation) and the trait anxiety (overall personality status) [22]. The Spielberger’s STAI is comprised of 40 questions. The first 20 questions are related to the state anxiety while the second 20 questions are about the trait anxiety. Each question of the questionnaire had four answer choices and the participants were asked to select the choice that best described the intensity of their feelings. To calculate the participant’s score in each of the two subscales, the sum of scores of all 20 questions for each subscale was calculated. Thus, the scores of each of the two subscales of anxiety namely the state anxiety and the trait anxiety could range from 20 to 80. Higher scores indicated higher anxiety. The validity and reliability of the Farsi version of this que-stionnaire have been previously confirmed by Shah Mansouri et al. [23].
Data were analyzed using SPSS version 23 (SPSS Inc., IL, USA) via t-test and Pearson’s test. P<0.05 was considered statistically significant.
3. RESULTS
A total of 89 patients with geographic tongue (20 males and 69 females) and 91 controls (13 males and 78 females) between 18 to 50 years were evaluated. The two groups were matched in terms of age, gender, marital status, level of education and socioeconomic status (P>0.05).
Of 89 patients, 23 complained of pain and burning sen-sation while 66 were asymptomatic.
Regarding the severity of condition (number of lesions), of 89 patients, 28 (31.5%) had one lesion (mild), 58 (65.1%) had 2-5 lesions (moderate) and 3 (3.4%) had 6 or more lesions (severe).
Considering the fact that the salivary level of alpha-amylase in patients was higher than that in controls, this difference did not reach statistical significance (t-test, P=0.767, Table 1).
| Group | Mean salivary level of alpha-amylase |
|---|---|
| Patient | 2488.9 ± 883.6 |
| Control | 2450.0 ± 835.1 |
| P-value | 0.767 |
Although the mean scores of STAI-T and STAI-S in geographic tongue patients were higher than those in controls, the differences were not significant either (t-test, P>0.05, Table 2).
| Group | Mean score of STAI-S | Mean score of STAI=T |
|---|---|---|
| Patient | 44.12±3.2 | 43.1±10.5 |
| Control | 42.6±9.6 | 42.1±10.1 |
| P-value | 0.358 | 0.527 |
The Pearson’s test revealed that increase in anxiety scores was significantly associated with an increase in the salivary level of alpha-amylase; however, no significant correlation was found between the salivary level of alpha-amylase and STAI-S and STAI-T scores (P>0.05, Table 3, Fig. 1).
| Correlation | Pearson’s correlation coefficient | P-value |
|---|---|---|
| Correlation of salivary level of alpha-amylase and STAI-S score | 0.096 | 0.258 |
| Correlation of salivary level of alpha-amylase and STAI-T score | 0.016 | 0.842 |
4. DISCUSSION
Geographic tongue is a common, benign lesion, which can considerably affect the physiological and social function of patients due to its specific characteristics [24]. Although this condition was identified about 150 years ago, its etiology is still unknown [5]. However, anxiety has been suggested as a possible etiologic factor playing a role in the occurrence of geographic tongue [10, 25].
This study showed that the mean salivary level of alpha-amylase in patients with geographic tongue was slightly, but not significantly, higher than that of controls. Thus, it does not seem that the salivary level of alpha-amylase can serve as a specific biomarker for this condition or could be suggested as a possible etiologic factor. To the best of authors’ knowledge, no previous study is available on the salivary level of alpha-amylase in geographic tongue patients to compare our results with their findings. However, some previous studies measured the salivary level of alpha-amylase in patients with other oral mucosal conditions. Cardoso et al. [21], showed that the salivary level of alpha-amylase was not significantly different in patients with aphthous ulcers and controls. Haririan et al. [26], showed that the salivary level of alpha-amylase was not significantly different in the three groups of patients with chronic periodontitis, aggressive periodontitis and controls. Although the aforementioned studies did not assess the correlation of the salivary level of alpha-amylase with geo-graphic tongue, they did not find a correlation between the salivary level of alpha-amylase and oral inflammatory conditions either, which was in line with our findings. How-ever, some differences exist in this respect. For instance, Nosratzahi et al. [20], demonstrated that the salivary level of alpha-amylase was significantly different in patients with burning mouth syndrome and controls.
The etiology of geographic tongue is not yet known. Several factors have been suggested as possible etiologic factors for this condition. Huamei et al. [27], stated that geographic tongue may be correlated with psychological stress and that the stress and tension may aggravate burning sensation in patients with geographic tongue.
In the present study, level of anxiety was evaluated using the Spielberger’s STAI, which has two subscales of state and trait and yields two final scores for level of anxiety of each individual [28]. In the present study, the mean scores of STAI-S and STAI-T were higher in geographic tongue patients than controls, but not significantly. Alikhani et al. [29], indicated that the level of anxiety of patients with geographic tongue was significantly higher than that of controls. Ebrahimi et al. [24], demonstrated that the level of anxiety of patients with geographic tongue was higher than that of healthy controls. These findings are different from our results, which may be due to differences in clinical characteristics of the study popu-lations and sample size of the two studies. Moreover, the difference in questionnaires and tools used for measurement of the level of anxiety may also be responsible for these diff-erences. Use of other questionnaires and tools for assessment of the level of anxiety in future studies may yield different results.
The current study revealed a direct correlation between the salivary level of alpha-amylase and STAI-S and STAI-T scores in the patient and control groups such that the salivary level of alpha-amylase was higher in those with higher anxiety score; however, this correlation was not significant. Cardoso et al. found no significant association between the salivary level of alpha-amylase and the anxiety score [21], which was in agreement with our findings.
Studies on the association of level of anxiety in patients with geographic tongue are limited. Several studies assessed the level of anxiety in patients with other oral conditions such as lichen planus, burning mouth syndrome and aphthous ulcers [20, 21, 30] and showed that the level of anxiety in these patients was significantly higher than that in controls. One important reason for the difference in level of anxiety of patients with geographic tongue compared to those with lichen planus, aphthous ulcers and burning mouth syndrome is the presence of clinical signs and symptoms such as pain and burning sensation in the latter conditions while the majority of geographic tongue patients evaluated in the current study were asymptomatic or had very mild symptoms. Obviously, clinical symptoms such as pain and burning sensation can cause problems in eating, deglutition, speech and daily routines and all these factors can lead to further anxiety and stress in these patients compared to healthy controls. This can be a reason for the absence of a significant difference in the level of anxiety of geographic tongue patients and controls. Absence of a significant difference in the level of anxiety of patients and controls in our study can also be due to the fact that dental patients are mostly anxious due to dental fear, which may result in higher than normal level of anxiety in the control group. This was an uncontrollable limitation of our study. Temporarily increased level of stress and anxiety in subjects presenting to a dental clinic can serve as a confounder, which can vary among different individuals.
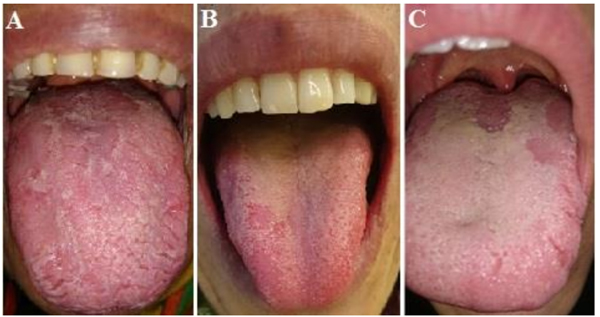
In total, the current results showed that despite a slightly higher level of salivary alpha-amylase and anxiety in geo-graphic tongue patients compared to controls, these diffe-rences did not reach statistical significance. However, the use of larger sample size and other anxiety screening tools may yield different results in future studies.
CONCLUSION
The results showed that anxiety can be viewed as an influential factor in the occurrence of geographic tongue; however, it is not the only influential factor in this respect. Salivary level of alpha-amylase is a minor factor in the assessment of patients with geographic tongue and cannot serve as a specific para-clinical biomarker for this condition.
ETHICS APPROVAL AND CONSENT TO PARTI-CIPATE
The study was approved by the Medical Ethics Committee of Isfahan University of Medical Sciences, Iran (code: 30406).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this study.
AVAILABILITY OF DATA & MATERIAL
Data sharing is not applicable in this study as no new datasets were generated in the research.
FUNDING
This work was supported by the vice chancellor for research of Isfahan University of Medical Science (NO: 396406).
CONFLICTS OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors thank the participants for taking part in this study.


