All published articles of this journal are available on ScienceDirect.
Alveolar Ridge Preservation: A Histomorphometric Analysis
Abstract
Objective:
This study presents the histomorphometric findings after tooth extraction with and without Alveolar Ridge Preservation (ARP) with a collagen cone filling the socket in combination with a collagen membrane covering the socket.
Materials and Methods:
In a controlled randomized clinical study, 10 patients were treated with the combination material after tooth extraction. In 10 patients, the extraction sockets were left to heal without further intervention. Soft tissue, new bone formation, bone quality and bone remodeling, blood flow vascularization, and inflammation were evaluated histomorphometrically. This was performed (semi-) quantitatively using a blinded protocol.
Results:
The statistical evaluation showed no significant difference for any parameter. When the combination material was used, more pronounced remodeling, increased osteoblast activity, and increased vascularization were demonstrated based on the histomorphometric findings. In contrast, there were reduced levels of osteogenesis and less mineralization. There was slightly more bundle bone in patients with ARP.
Conclusion:
The histomorphometric analysis of ARP with a combination material consisting of a collagen cone and a collagen membrane showed no significant differences in terms of new bone formation and bone quality. Descriptively, however, different manifestations were seen that might benefit from being documented using larger samples and being tested for clinical relevance.
1. INTRODUCTION
Tooth extraction results in resorptive changes of the alveolar process [1]. In particular, there is a significant loss of bone volume on the buccal aspect of the empty socket, with an ultimate loss of volume of the alveolar process [2]. At 4-8 weeks after tooth removal, new bone forms, starting from initial islands of bone within the connective tissue. At 10-20 weeks, more mature trabecular structures can be recognized, and the number of osteoblasts is reduced [3]. The remodeling associated with bone regeneration, from connective tissue to mineralized new bone, takes place within a time interval whose beginning, end, and hence duration are unpredictable [4].
The bone loss after extraction causes horizontal ridge resorption by an average of 3.8 mm and vertical ridge resorption by an average of 1.2 mm during the first 6 months [5, 6]. Measures to preserve the dimensions of the alveolar bone during the first weeks after tooth extraction have a positive effect on soft-tissue profile maintenance [7, 8].
The loss of hard and soft tissue and the different interindividual maturation over time of the new bone influence the implantological treatment at the site.
An approach to stabilizing the bone and slowing the resorptive processes consists of introducing filler materials into the empty socket [9, 10]. In addition to autologous bone, allogeneic, xenogeneic, or synthetic bone replacement materials are available for ARP. Different approaches to ARP have reduced the loss of hard and soft tissues, but they did not completely prevent resorption [11]. Such approaches have included the introduction of freeze-dried demineralized bone, bioactive glasses, or hydroxyapatite into extraction sockets. It was found that bone will be lost even when filler materials are introduced, although less so than after extraction without ARP [12]. The best clinical results were obtained with FDBA (freeze-dried bone allograft) [9, 13].
Table 1 provides an overview of histological analyses from prospective studies found during a systematic literature search. The literature overview is based on a PubMed search performed on January 1, 2016 using the search term: (clinical AND (trial OR study OR systematic review) AND (ARP OR “alveolar ridge preservation” OR “socket preservation” OR (tooth OR teeth AND (ridge preservation OR socket preservation) AND histol*)). To be included, studies had to comprise at least 10 cases per group and a control group with untreated extraction sockets. The overview shows the level of heterogeneity with regard to time of the sampling, test materials, and the sealing of the sockets.
| Author |
Study Design Number of Cases |
Time of Examination | Sex | Age | History of Perio | Smoking |
Jaw: Max./ Mand./ Both |
Location: Inc/PM/M | Reason for Extraction | Socket Classification |
|---|---|---|---|---|---|---|---|---|---|---|
| Barone et al. (2008) | RCT / 40 | 7–9 months | 16 male, 24 female |
26–69 y | Not described | Heavy smokers (> 10 cig/d) excluded |
both | Inc/PM | Not described | Only alveoli with 4 intact walls |
| Barone et al. (2017) | RCT / 90 | 3 months | 36 male, 54 female |
25–70 y, mean 47.4 y | Not described | Heavy smokers (> 10 cig/d) excluded | both | PM/M | Decay, endodontic failure, fracture | Y, according to Juodzbalys (2008) |
| Cardaropoli et al. (2012) |
RCT / 48 | 4 months | 24 male, 17 female |
24–71 Y, mean 47.2 y | Acute perio excluded | Heavy smokers (> 10 cig/d) excluded | both | PM/M | root fracture, periodontal involvement, endodontic treatment failure. decay | three intact walls and at least 80% of the fourth wall intact |
| Casado et al. (2010) | CT / 15. no histology in the control group |
4 months | No data | No data | Not described | Not described | both | Inc/PM/M | Not described | Intact alveoli only |
| De Coster et al. (2011) |
CT / 10 (23 sockets) | 6–74 weeks | 8 male, 2 female |
41-81 y | Not described | Smokers and non-smokers | both | Inc/PM/M | Caries, perio, fracture | Intact buccal bone |
| Crespi et al. (2009) | RCT / 45 | 3 months | 8 male, 7 female |
28–-71 y, mean 51.3 y | Excluded | Non-smokers | both | PM/M | Not described | 3-walled, loss of the buccal bone wall |
| Nahles et al. (2013) | RCT / 33 | 25 sites: 4 weeks, 40 sites: 12 weeks |
18 male, 15 female |
30–73 y, mean 54.5 y | Acute PAR and with PPD > 5 mm excluded | Non-smokers | both | Inc/PM/M | Not described | 4 walls |
| Pelegrine et al. (2010) |
CT / 13 | 6 months | 7 male, 6 female |
28–70 y, mean 47.5 y |
Not described | Non-smokers | OK | Inc | Not described | Not described |
| Barone et al. (2008) | Y | AB (4 days); CHX rinse (3 weeks) | Xenogeneic | Corticocancellous porcine bone | MP3; OsteoBiol, Coazze, Italy | Test group: Y; control group: N | Evolution collagen membrane; OsteoBiol, Coazze, Italy | Yes, more trabecular bone and less connective tissue in the test group | ||
| Barone et al. (2017) | N | AB (5 days), CHX rinse (3 weeks) | Xenogeneic | Test 1: collagenated cortico-cancellous porcine bone; test 2: cortical porcine bone | Test 1: MP3;OsteoBiol, Coazze, Italy, Test 2: Apatos; OsteoBiol, Coazze, Italy |
Test group: Y; control group: N | Evolution collagen membrane; OsteoBiol, Coazze, Italy | Yes, greater mineralization in the cortical-bone test group | ||
| Cardaropoli et al. (2012) | N | AB (6 days); + CHX rinse (duration not stated) | Xenoeneic | Bovine bone mineral | Bio-Oss Collagen | Y | Only test group with porcine collagen membrane (Bio-Gide) | Yes, more new bone in test group | ||
| De Coster et al. (2011) |
Y | Not described | Alloplastic | 60% hydroxyapatite, 40% β-tricalcium phosphate |
BoneCeramic (Straumann AG) | Y | Primary wound closure, no membrane | Not reported | ||
| Crespi et al. (2009) | N | Single shot of Amoxicillin 1 g, 1 h pre-op | Alloplastic | 15 magnesium-enriched hydroxyapatite (MHA), 15 calcium sulfate (CS) | MHA: SINTlife; Finceramica, Faenza, Italy; CS: Easy Set; Sweden-Matina, Due Carrare, Italy | Y | Y, with collagen membrane | Yes, with vital bone and connective tissue | ||
| Nahles et al. (2013) | N | Not described | Xenogeneic | Bovine granules and porcine collagen | Bio-Oss Collagen | N | N | No significant differences at the histomorphometric level | ||
| Pelegrine et al. (2010) |
Y | Not described | Autologous | Iliac crest bone marrow | N/A | Y | Sutured after periosteal slit | No, no significant differences |
In various studies on the incorporation of bone replacement materials into extraction sockets, no significant difference in bone formation was demonstrated at the histological level, but there was a positive clinical effect in terms of volume preservation of the alveolar bone [14-19]. In contrast, a comparative study of Demineralized Freeze-Dried Bone Allograft (DFDBA) and mineralized Freeze-Dried Bone Allograft (FDBA) used in ARP, while revealing no difference in the size of the alveolar bone, histologically showed significantly more vital bone when DFDBA was used [20]. This positive effect on bone regeneration has also been reported in other studies [21, 22].
Studies also showed that primary wound closure after extractions led to reduced absorption of the alveolar bone, but that this effect can be similarly demonstrated when the wound is covered with a membrane [23, 24]. The explanation that was cited was the protective stabilization of the blood coagulum. On the other hand, when introducing various filler materials into the empty socket, collagen resulted in demonstrably reduced absorption [25]. Animal experiments showed that the introduction of a collagen cone combined with a collagen membrane significantly reduced bone resorption compared to untreated extraction sockets [26].
A new, completely resorbale material is available with the combination material Parasorb Sombrero® (Resorba, Nürnberg, Germany), which combines a collagen cone containing equine collagen fibrils of 32.2 mg and an equine collagen membrane that is not chemically cross-linked. Both materials have been combined into a single product to facilitate simple and quick deployment. To date, no adequate clinical human studies have been performed on this material [26, 27].
The objective of the present study is to examine, using histomorphometric methods, the clinical results for bone preservation using the described combination material compared to untreated extraction sockets.
2. MATERIALS AND METHODS
The study was conducted as a prospective controlled randomized clinical study according to the Declaration of Helsinki. The procedure and all the materials used were submitted to the relevant Ethics Committee: Ethics Committee of the University of Ulm and approved (No. 337/12, approved Feb. 13, 2013).
The study participants were informed about the study before their participation, both orally and in writing, and gave their written informed consent.
2.1. Study Patients
Twenty patients who required extraction of a maxillary tooth to be subsequently replaced by a fixed implant-supported restoration participated in the histological part of the overall study that included 60 patients [28]. They were the first 10 patients of the test and control groups in consecutive order.
In 10 patients, a combination material consisting of a collagen cone and a collagen membrane (Parasorb Sombrero®; Resorba) was inserted into the extraction alveoli after extraction. In the other 10 patients, wound healing was allowed to proceed without further intervention.
The inclusion criterion was at least 1 maxillary tooth requiring extraction. The extraction could be indicated for periodontal reasons or because of advanced tooth destruction by caries or trauma.
A prerequisite for inclusion in the patient group was that a tooth or an existing implant was present immediately adjacent to the tooth to be extracted.
Patients meeting at least 1 of the following criteria were excluded:
- Age under 18 years or legally incompetent
- Recognizable additional primary need for augmentation due to greatly advanced vertical bone defects.
- An intraoral situation that would make the insertion of the implant with the aid of a drilling template impossible (insufficient mouth-opening capacity)
- Heavy smoking (more than 10 cigarettes/day)
- Use of bisphosphonates
- Pregnancy
- Alcohol or drug abuse
- Infectious disease such as hepatitis or HIV/AIDS
- Patients with uncontrolled severe diabetes mellitus; The HbA1c long-term blood glucose indicator must be less than 6.7%.
The patients had previously approved of having their respective missing teeth replaced by an implant.
2.2. Treatment Protocol
All interventions and the follow-ups were performed in the private practice of the first author. All patients were treated exclusively by the same dentist (SIS).
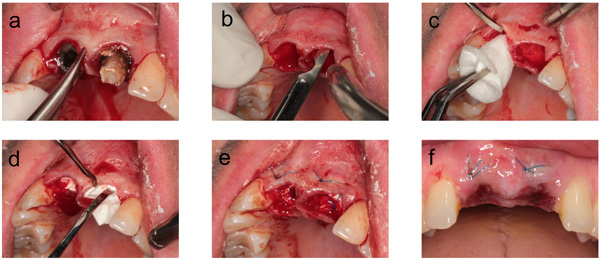
Local anesthesia was performed with Ultracain DS 1: 200,000 (Sanofi Aventis, Frankfurt, Germany).
Molars were decapitated and the roots separated with a diamond disk in a dental turbine.
A gentle extraction was performed using periotomes and removing the tooth with a dental forceps following complete mobilization (Fig. 1a). The extraction socket was then carefully curetted.
No further socket-related measures were carried out in the control group. A sterile bite swab was inserted, and the patient was asked to loosely bite on it for 20 minutes duration and then to remove it.
In the intervention group, the collagen cone was introduced according to the manufacturer's instructions. A circular supraperiosteal pocket of coronal soft tissue was prepared (Fig. 1b). This soft tissue was not mobilized, meaning that the socket received no primary mucosal seal. The collagen plug, trimmed to the size of the socket, and the trimmed membrane were introduced into the socket without pressure (Figs. 1c, d). To prevent the plug from being ejected from the socket, the dentist placed a cruciate mattress suture with Resolon 4-0 (Resorba), a monofilament polyamide-6 thread (Fig. 1e).
After 1 week, the wounds were visually inspected in all patients. In the patients of the intervention group, the sutures were removed at the same time (Fig. 1f).
After the extraction, all patients received instructions for the next 24 hours. Specifically, patients were instructed to avoid the following:
- Eating while the effect of the anesthesia was still noticeable
- Consuming alcohol, coffee or caffeinated beverages, and cigarettes or other tobacco products
- Rinsing the extraction wound (this was to preserve blood coagulation)
- Manipulating the wound manually (for example, pulling on the lip, massive cleaning of the wound)
Patients were prescribed 600 mg of ibuprofen for pain reduction, to be taken as individually needed. No prophylactic antibiotic was prescribed.
A temporary interim denture was provided only where essential (for aesthetic reasons in the anterior region or for functional reasons in the case of multiple tooth loss), and then only at the request of the patient.
The implants were placed 11 ± 1 weeks after tooth removal, following virtual planning using an implant planning software (SMOP; Swissmeda, Zürich, Switzerland). The planned implant position was transferred by means of a printed surgical guide.
The biopsy was performed by means of a trephine drill with an external diameter of 3.2 mm, an internal diameter of 2.6 mm and a length of 5-10 mm, with the drill direction being determined by the surgical guide. No pilot drill was required.
2.3. Histomorphometry
Each biopsy was fixed by immersion in 4% buffered formaldehyde at Room Temperature (RT) for at least 1 day and subsequently decalcified for about 3 weeks in 4.1% disodium Ethylenediaminetetraacetic Acid (EDTA) solution, which was changed every 24 hours. After hydration, tissues were dehydrated in an ascending series of ethanol solutions and embedded in paraffin. Serial sagittal sections of 2-3 µm were cut, and representative slides were stained with Hematoxylin-Eosin (HE) and Masson-Goldner trichrome for an overview. In addition, PAS staining was performed for the histochemical detection of glycosaminoglycans and glycoproteins, and to identify osteoclasts, selected tissue sections were stained to demonstrate Tartrate-Resistant Acid Phosphatase (TRAP).
The histomorphometric preparation and evaluation was carried out in cooperation with the Laboratory for Basic Oral Biological Research at the University of Bonn.
The samples were evaluated for the following parameters:
- Osteogenesis
- Remodeling
- Osteoblast activity
- Discernible calcification/mineralization
- Detection of bundle bones in the alveoli
- Vascularization of the alveoli
- Inflammation

A semiquantitative evaluation was carried out based on the qualitative characteristics. A preliminary examination determined whether the individual parameters could infact be evaluated. If so, the evaluation was carried out semi-quantitatively, yielding a yes/no result. Each possible evaluation resulted in a valid data record. The semiquantitative scoring system was based on the specifications of the guidelines of the International Organization for Standardization (ISO) 10993-6 Biological evaluation of medical devices part 6: Tests for local effects after implantation [29] (Figs. 2-4).
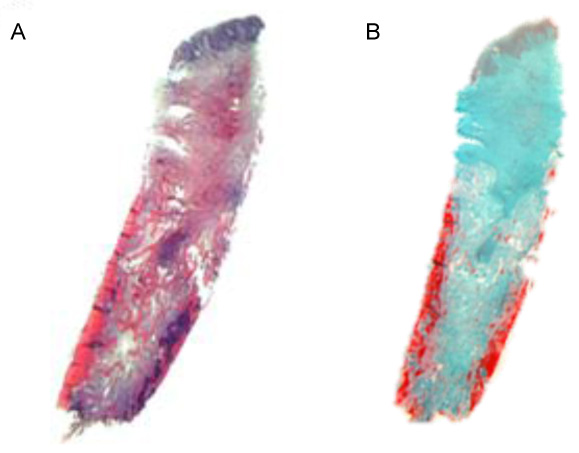
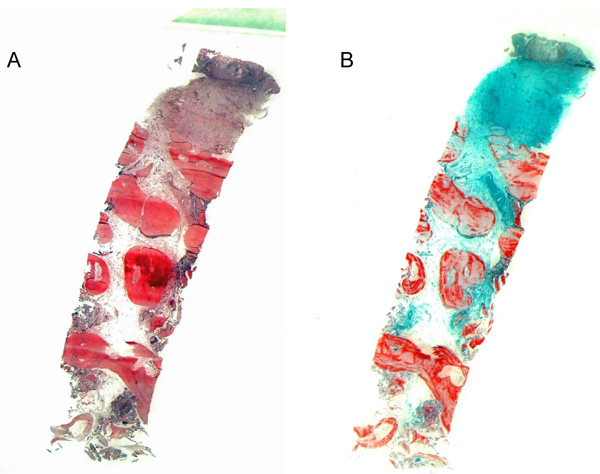
The evaluation levels were 0 = none; 1 = low; 2 = pronounced; 3 = very pronounced.
When assessing necrosis or signs of inflammation, the location was also recorded as gingiva, connective tissue, or bone.
2.4. Estimated Sample Size
Due to the lack of clinical data, no a priori sample size estimate could be obtained. The number of cases with 10 test and 10 control samples was based on the specifications of the ISO10993-6 [29]. This study was therefore carried out as an exploratory study. We intended to perform a post-hoc power analysis to provide a basis for future comparative studies.
2.5. Randomization
A randomization list was created for the overall study that included 60 patients (Institute of Epidemiology and Medical Biometry, University of Ulm, Germany). Assignment to the various groups was made in 6 layers. The data were stratified as follows:
- By sex (2 groups: male or female)
- By region of the test tooth (3 groups: anterior, premolar, molar)
The study director or a person authorized by him, instructed the treatment center by fax as to the type of treatment to be performed according to the randomization list.
2.6. Blinding
The laboratory received the samples in an anonymous form. The results were recorded on dedicated forms. The blinding was maintained until the samples had been completely prepared, analyzed, and documented, and taken to a different place with a different operator than the researcher responsible for the histomorphological evaluation.
2.7. Statistical Analysis
For the metric target variables, the minimum, median, and maximum were reported. Nominal and ordinal features were described with their absolute and relative frequencies.
Differences between the test and control group were tested using the Wilcoxon rank-sum test. Given the exploratory nature of this study, all statistical results are hypothetical in nature and should not be interpreted as conclusive. All statistical tests were carried out at level α = 0.05 (double-sided). No adjustment was made for multiple testing. The post-hoc power and sample-size estimates were given for the Wilcoxon rank sum test. The post-hoc sample-size estimate was based on a pre-set power of 80% and the first error of α of 5%.
The evaluation was performed with SAS® Version 9.4 and IBM SPSS Statistics 21. The calculation of the power analysis and the sample-size was carried out using the Proc Power feature of SAS® Version 9.4.
3. CRITICAL ASSESSMENT OF THE RESULTS
3.1. Patients
All patients were treated according to the clinical protocol. There were no postoperative complications. All included patients completed the study.
The examinations took place in the period between June 4 and December 3, 2013.
The study included 10 female patients and 10 male patients. The mean age of the patients was 46.6 (21.9-71.4) years.
| Region | Test Group | Control Group | Total |
|---|---|---|---|
| Anteriors | 5 | 7 | 12 |
| Premolars | 4 | 2 | 6 |
| Molars | 1 | 1 | 2 |
In the test group, the mean age was 44.3 (21.9-71.4) years; in the control group, it was 48.8 (33.1-58.3) years. The randomized distribution of the teeth is shown in Table 2.
The results are presented in Table 3. The Wilcoxon rank-sum test showed no significant difference for any parameter. When the combination material was used, more pronounced remodeling, increased osteoblast activity, and increased vascularization were demonstrated descriptively. In contrast, there were reduced levels of osteogenesis and less mineralization. There was slightly more bundle bone in patients with ARP.
| Parameters | Procedure | Valid Records | Minimum | Median | Maximum | Hypothesis Test |
|---|---|---|---|---|---|---|
| Osteogenesis | with | 10 | 1.00 | 2.00 | 3.00 | 0.51 |
| without | 9 | 1.00 | 2.00 | 3.00 | ||
| Remodeling | with | 10 | 0.00 | 1.00 | 2.00 | 0.60 |
| without | 9 | 0.00 | 0,00 | 2.00 | ||
| Osteoblast activity | with | 10 | 1.00 | 2.00 | 2.00 | 0.26 |
| without | 9 | 1.00 | 2.00 | 2.00 | ||
| Mineralization | with | 10 | 0.00 | 1.00 | 2.00 | 0.12 |
| without | 9 | 0.00 | 2.00 | 2.00 | ||
| Bundle bone | with | 10 | 0.00 | 0.00 | 2.00 | 0.34 |
| without | 9 | 0.00 | 0.00 | 1.00 | ||
| Vascularization | with | 10 | 1.00 | 2.00 | 3.00 | 0.41 |
| without | 9 | 1.00 | 2.00 | 2.00 | ||
| Inflammation | with | 10 | 0.00 | 1.00 | 2.00 | 0.50 |
| without | 10 | 0.00 | 1.00 | 2.00 |
The descriptive analysis of the regions where inflammatory processes were detected was the same for both groups; the inflammatory processes were mostly located in the connective tissue.
The highest statistical power analyzed in the samples was 23.5% for osteoblast activity and 20.3% for mineralization. The post-hoc sample size estimate exhibited a considerable spread between parameters. Here, sample sizes of 96-434 patients were calculated (Table 4).
| Parameter | Power (1-β err. prob.) |
Estimated Sample Size Total Sample Size |
|---|---|---|
| Osteogenesis | 0.114 | 276 |
| Remodeling | 0.104 | 434 |
| Osteoblast activity | 0.235 | 96 |
| Mineralization | 0.203 | 130 |
| Bundle bone | 0.186 | 128 |
| Vascularization | 0.150 | 170 |
| Inflammation | 0.129 | 286 |
4. DISCUSSION
The different time points of the histological examination make a comparison with other studies difficult. For example, Barone et al., (2008) demonstrated statistically significant differences between ARP and xenografts compared to untreated extraction sockets after 7 months of mineralization [23]. But even longer healing periods after introducing different bone replacement materials may not lead to significant differences between groups at the histomorphometric level [30]. The protocol of delayed immediate placement until 3 months, as prescribed for the present study, does not show a sufficient difference in mineralization due to the temporal sequence of the mechanisms involved in regeneration [4]. The effect of ARP has so far been examined after a period of 3 to 6 months [1, 31, 32].
Furthermore, a direct comparison with published studies is hardly possible for a number of methodological reasons. Histological examinations were involved in a very small number of cases [33-35] and should rather be considered case reports. Another limitation is that various studies included no control group in which bone healing took place without therapeutic intervention [15, 16, 20, 36]. An evaluation of ARP at the clinical and histomorphometric levels can be put in perspective if, in addition to the test group, a control group with untreated sockets is available for comparative evaluation after extraction.
Patient selection, too, leads to different results. For example, bone regeneration in patients with periodontal disease is much slower and less predictable than in patients without periodontal disease [37]. The age of the patient also has an influence on the healing process after tooth extraction. Angiogenesis and osteogenesis are delayed in aging patients [38]. In the study presented here, the patients of the test group were 4.5 years younger than those of the control group.
This means that this study allows no differentiation of the effects caused by obturating the empty socket and those caused by the crestal wound surface being covered with a membrane. The benefits of the barrier function of membranes are controversial [39]. In various studies, however, membranes have been shown to increase bone regeneration [40] or reduce resorption of the coronal bone [41].
The power analysis calculated in this study showed that much larger sample sizes are needed due to the small differences and the low effect sizes in the semi-quantitative evaluation of the different characteristics. One reason for this is the use of non-parametric tests with ordinal target variables and few degrees of expression. But using more degrees of expression would suggest a level of accuracy that is not real.
For comparative studies at the histological level, no post-hoc power analyses or sample-size estimates were found. In reports on clinical studies on ARP, only a few post-hoc power analyses were published. The required number of cases is between 10 and 18 for significant differences in clinical parameters of bone degradation to become discernible at a statistical effect size of 80% to 88.5% [20, 42-46]. Table 1 show that the studies with high sample sizes can also detect statistically significant differences even for histological parameters.
The delayed osteogenesis and mineralization observed when using a collagen material was also corroborated by a similar prospective histological study [47] where Bio-Oss collagen was used as a filler and compared to non-augmented sockets. The formation of new bone was 25% in the test group, compared to 44% in the control group. The sampling time-12 weeks after extraction was also comparable with the present study. An explanation might be that the introduction of an extraneous material causes the processes of osteogenesis to be delayed, since the conversion and degradation of the filler material take precedence.
The trend towards more pronounced vascularization when using the collagen material can have a positive effect on the biological processes during wound/socket healing. The vascular density during alveolar healing starts receding from week 2 to 4 post-extraction [4]. If appropriate measures could be taken to promote angiogenesis, this would also benefit osteogenesis, which is dependent on vascularization. There could also be positive effects in patients with age-related reduced angiogenesis [38].
However, a possible influence or clinical relevance of the different effects of the combination material on the formation of new bone can be neither proved nor disproved with the methods employed in the present study. Further investigations are required to show whether postponing implant placement beyond the 3 months provided by our delayed immediate placement protocol are beneficial when ARP is performed.
CONCLUSION
For studies with histomorphometric targets, sample-size estimates will continue to be of interest. The number of cases shown to be necessary are ideally achieved by multicentric studies.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by Ethics Committee of the University of Ulm and approved (No. 337/12, approved Feb. 13, 2013). German Clinical Trials Register and the International Clinical Trials Registry Platform of the WHO: DRKS00004769 (pre-study), date of registration: Feb. 28, 2013; and DRKS00005978 (main trial), date of registration: Nov. 09, 2015.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Consent was obtained from the participants before their participation.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEEMENTS
SIS made contributions to the acquisition of funding, conception, and design, as well as substantial contributions to the coordination of the study, clinical performance, acquisition of data, and drafting the manuscript. WG made contributions to the conception and design of the study, and funding of the study. WG also made substantial contributions to the conception and statistical design of the study and was involved in drafting the manuscript. HR made substantial contributions to the conception, design, and coordination of the study and was involved in drafting the manuscript. RL made substantial contributions to the acquisition of funding, conception, design, and coordination of the study and was involved in drafting the manuscript. All authors read and approved the final manuscript. All authors agree to be accountable for all aspects of the work by ensuring that questions related to the accuracy or integrity of any parts of the work are appropriately investigated and resolved.


