All published articles of this journal are available on ScienceDirect.
Dental Implant Rehabilitation in Patients Suffering from Mucocutaneous Diseases: A Systematic Review and Meta-Analysis
Abstract
Objective:
Dental implantations are widely used for oral rehabilitation of edentulous patients. Despite high success rate, there are some risk factors that have been associated with failures. Oral mucocutaneous diseases are one of these risk factors for implant insertion due to the immunosuppressive therapy.
There are limited studies that have dealt with the subject of dental implantology in oral mucosal disorders mainly with patients with oral lichenplanus, pemphigoid, pemphigus vulgaris, and systemic lupus erythematosus. In order to assess the result of implantations in such patients, we have reviewed the studies.
Materials and Methods:
We searched PubMed, Science Direct, and Cochrane databases for articles published from Jan 2000 to Dec 2017, using key search word “dental implants”, “oral lichen planus”, “pemphigoid”, “pemphigus vulgaris” and ”systemic lupus erythematosus”.
Results:
The random effects analysis result shows overall failure rates of 22% in patients with oral lichen planus. A systematic review revealed some failures that are not definitely related to these diseases.
Conclusion:
Due to the lack of adequate studies, a meta-analysis was only possible for oral lichen planus. Presently, there is no definite guideline regarding the placement of implant in patients suffering from mucocutaneous diseases; nevertheless, we should always consider that these patients are specific cases and need more attention in the first step of treatment and follow-ups. So there is a need to further clinical studies in order to evaluate more risk factors accurately and make a definitive conclusion.
1. INTRODUCTION
Dental implantations are widely used for oral rehabilitation of both partly and fully edentulous patients worldwide and offer a survival rate of around 90-95% after 10 years [1-6]. The survival rate exceeding 95% in patients without any oral or systemic complications [7, 8].
Despite high success rate, there are some failures. The risk factors that have been associated with failures include the quality and quantity of the remaining alveolar bone, environmental factors such as smoking, many local and systemic diseases including osteoporosis, diabetes, immunosuppression, bone disease, gastrointestinal problems, leukemia, and other congenital diseases [9-19].
Oral mucosal disorders are one of these risk factors for implant insertion due to their possible effects on oral hard and soft tissues [20]. There are few studies that have dealt with the subject of dental implantology in oral mucosal disorders and mainly with patients with Oral Lichenplanus (OLP), pemphigoid, Pemphigus Vulgaris (PV), and Systemic Lupus Erythematosus (SLE).
A short introduction of clinical features of oral lichenplanus, pemphigoid, pemphigusvulgaris, and systemic lupus erythematosus are as follows:
OLP is a chronic inflammatory mucosal disease of unknown exact etiology [21, 22]. OLP usually manifests in age groups over 50 years old, more frequently in women than men [23-26]. The most common affected sites are oral mucosa, tongue, and gums, where the disease presents with white striations, papules, plaques, erythema, erosions, or blisters [26, 27].
As a matter of fact, patients with OLP complain of burning mouth, erosions, and ulcerations of the oral mucosa [28-30]. So dental treatment of such patients includes avoidance or removal of factors irritating the mucosa [31, 32]. Due to the friability of oral mucosa, implant-supported prosthesis is one of the best treatment plans in such patients [33, 34].
Pemphigoid is a chronic autoimmune vesiculobullous disorder. Mucous membrane pemphigoid can affect the mucous membrane of oral cavity, nasopharynx, larynx, esophagus, conjunctiva, genitourinary tract, and anus with rare skin involvement. It is one of the major causes of gingival desquamation and is characterized by subepithelial bullae [35, 36].
PV is the most common type of pemphigus. PV is a chronic mucocutaneous vesiculobullous disease with a peak incidence between the fifth and sixth decades of life [37, 38]. It causes erosions, blisters, and ulcers on the oral mucosa and skin [39, 40].
SLE is a multisystemic autoimmune disease with unknown etiology. It has periods of remission and flares and predominantly occurs in women. Oral lesions are seen in more than 40% of the affected patients, which can involve the hard palate, lips, and buccal mucosa. It is characterized by tissue damage resulting from the deposition of immunoglobulins in the form of antigen-antibody complexes [41-43].
Current treatments of these oral mucosal disorders are based on the long-term use of topical and systemic steroids or other immunosuppressive drugs. Immunosuppressive drugs may increase the risk of infection and delayed healing, which is a challenge during implant insertion [39, 40].
Although the prevalence of these diseases seems to be low, the onset of the symptoms mostly reveals in adults aged over 50 years who are in need of dental prosthetic treatment [44]. The quality of life in these patients is often poor and dental implantation could improve speaking, chewing, and swallowing.
In spite of the wide use of dental implants, there are few studies about implant rehabilitation in patients with mucocutaneous disorders and also there are no guidelines and/or recommendations for a treatment plan for such patients. Due to the increasing success of dental implants in such patients, the spectrum of contraindications has been greatly modified [22]. But there is still a possibly higher risk of peri-implantitis and early implant failure in such patients. The aim of this meta-analysis and systematic review is to understand the implant success rate in such patients.
2. MATERIALS AND METHODS
A systematic literature search was performed using electronic databases (Medline/PubMed, Cochrane, Science Direct). Search strategy focused on the combinations of MeSH terms and included English language articles on clinical studies, case series, case reports, and review articles describing the outcome of dental implantations in patient with mucocutaneous diseases. The population comprised patients suffering from OLP, pemphigoid, PV, and SLE undergoing implant prosthetic treatment.
Search term combinations concerning OLP were (lichen planus[MeSH Terms]) or (lichen planus, oral [MeSH Terms]) or (oral lichen planus [MeSH Terms]) and (“2000/01/01”[PDat]: “2017/12/31”[PDat]) and (dental implant[MeSH Terms]) or (dental implantation, Osseointegrated[MeSH Terms]) or (dental implantation[MeSH Terms]) or (dental implantation, subperiosteal[MeSH Terms]) or (Dental Implantation, Endosseous[MeSH Terms] or (dental implants[MeSH Terms]) and (“2000/01/01”[PDat]: “2017/12/31”[PDat]).
For pemphigoid, the following search term combinations were used: (benign mucosal pemphigoid[MeSH Terms]) or (benign mucosal pemphigoids[MeSH Terms]) or (benign mucous membrane pemphigoid[MeSH Terms]) or (mucosal pemphigoid, benign[MeSH Terms]) or (bullous pemphigoid[MeSH Terms]) or (Mucosal pemphigoids, benign[MeSH Terms]) or (mucous membrane pemphigoid, benign[MeSHTerms]) or (pemphigoid[MeSH Terms]) or (pemphigoid, benign mucosal[MeSH Terms]) and (“2000/01/01”[PDat]:”2017/12/31”[PDat]) and (dental implant[MeSH Terms]) or (dental implantation, osseointegrated[MeSH Terms]) or (dental implantation[MeSH Terms]) or (dental implantation, subperiosteal[MeSH Terms]) or (Dental Implantation, Endosseous[MeSH Terms]) or (dental implants[MeSH Terms]) and (“2000/01/01”[PDat]: “2017/12/31”[PDat]).
Search term combinations for PV were (dental implant[MeSH Terms]) or (dental implantation, osseointegrated[MeSH Terms]) or (dental implantation[MeSH Terms]) or (dental implantation, subperiosteal[MeSHTerms]) or (Dental Implantation, Endosseous[MeSH Terms] or (dental implants[MeSH Terms]) and (“2000/01/01”[PDat]: “2017/12/31”[PDat]) and (pemphigus[MeSH Terms]) or (foliaceus, pemphigus[MeSH Terms]) or (pemphigus vulgaris[MeSH Terms]) or (benign chronic pemphigus[MeSH Terms]) or (benign familial pemphigus[MeSH Terms]) or (familial benign chronic pemphigus[MeSH Terms]) or (familial pemphigus, benign[MeSH Terms]) or (pemphigus foliaceus[MeSH Terms]) or (pemphigus foliaceus antigen[MeSH Terms]) or (pemphigus, benign familial[MeSH Terms]) and (“2000/01/01”[PDat]: “2017/12/31”[PDat]).
For SLE, the search combinations were (cutaneous lupus erythematosus[MeSH Terms]) or (discoid lupus erythematosus[MeSH Terms]) or (lupus erythematosusdisseminatus[MeSH Terms]) or (lupus erythematosuspanniculitides[MeSH Terms]) or (lupus erythematosusprofundus[MeSH Terms]) or (lupus erythematosus, chronic cutaneous[MeSH Terms]) or (lupus erythematosus, cutaneous[MeSH Terms]) or (lupus erythematosus, cutaneous, chronic[MeSH Terms]) or (lupus erythematosus, cutaneous, subacute[MeSH Terms]) or (lupus erythematosus, discoid[MeSH Terms]) or (lupus erythematosus, subacute cutaneous[MeSH Terms]) or (lupus erythematosus, systemic[MeSH Terms]) or (neuropsychiatric systemic lupus erythematosus[MeSH Terms]) or (neuropsychiatric systemic lupus erythematosus[MeSH Terms]) or (panniculitides, lupus erythematosus[MeSH Terms]) or (panniculitis, lupus erythematosus[MeSH Terms]) or (systemic lupus erythematosus[MeSH Terms]) or (central nervous system systemic lupus erythematosis[MeSH Terms]) or (systemic lupus erythematosis, central nervous system[MeSH Terms]) and (“2000/01/01”[PDat]: “2017/12/31”[PDat]) and(dental implant[MeSH Terms]) or (dental implantation, osseointegrated[MeSH Terms]) or (dental implantation[MeSH Terms]) or (dental implantation, subperiosteal[MeSH Terms]) or (Dental Implantation, Endosseous[MeSH Terms] or (dental implants[MeSH Terms]) and (“2000/01/01”[PDat]: “2017/12/31”[PDat]).
Review articles and studies that included another disease more than OLP, Pemphigoid, Pemphigus Vulgaris, and SLE were excluded. Reference lists of all retrieved articles were also screened for further studies that could be in accordance with the inclusion criteria.
Three reviewers independently evaluated all of the abstracts according to the inclusion criteria and after screening, the publications that were matched to the purpose of the research were selected.
Data were extracted on the type of the study, number of patients who suffered from the diseases and had dental implants, total number of implants, failure rate, age of patients, type of diseases, and the months of follow up.
A pooled random-effects meta-analysis was conducted using MedCalcstatistical software version 16.4.3 (MedCalcSoftware bvba, Ostend, Belgium; https://www.medcalc.org; 2016)” and non-weighted prevalence rates were calculated. The I2 (Higgins JPT, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ 2003;237:557-60.) value was calculated and tested by Cochran's Q test to assess the degree of inconsistency in the results of the studies. The value of I2 is expressed as a percentage of the total variation across studies that is attributed to heterogeneity rather than chance (Fig. 1). To evaluate publication bias (bias refers to a tendency to publish results that are significant) funnel plot (Egger et al., 1997, Egger, Matthias, et al. “Bias in meta-analysis detected by a simple, graphical test.” Bmj 315.7109 (1997): 629-634.), was applied.
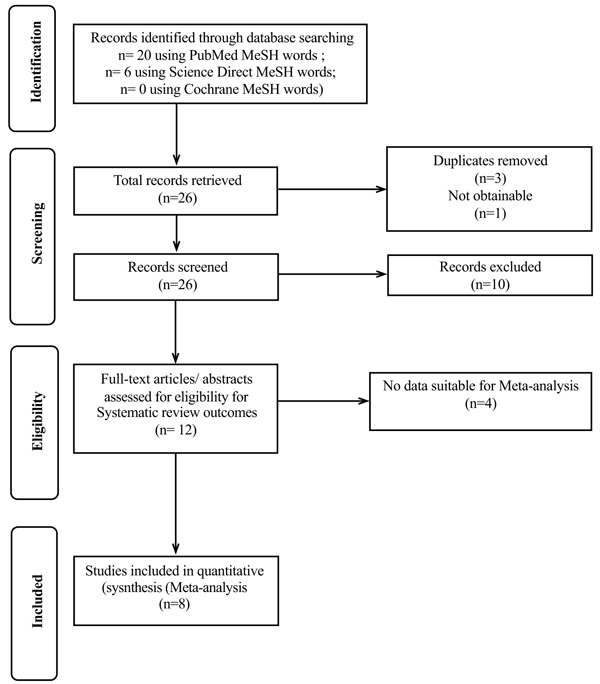
3. RESULT
The present study consisted of 12 articles, including review articles, case reports and case series, retrospective and prospective controlled studies, and cross-sectional studies. Among these articles,10 was related to OLP, 1 to PV, and 1 to SLE. The extracted data of each article are shown in Table 1.
| Date of Publication | Authors Name | Study Type | Number of Patients with OLP & Dental Implants | Total Number of Implants | Failure Rate | Age of Patient | Type of Disease | (Mean) Follow up (months) | Type of Implant Supported Prosthetic |
|---|---|---|---|---|---|---|---|---|---|
| 2014 | Lopez-Jornet et al. |
Cross sectional | 10 female 6 male |
56 | n.a | Range 44-76 64.5(Mean) |
OLP | Range 12-120 42 (Mean) |
3 over dentures 13 fixed partial prosthetic |
| 2006 | Reichart et al. | Report of 3 cases | 3 female | 10 | 0% | 63,68,79 70(Mean) |
OLP | 156(n=4), 36(n=2), n.a.(n=4 implant) | Fixed partial prosthetic |
| 2012 | Hernandez et al. |
Prospective-controlled study | 14 female 4 male |
56 | 0% | 53.5 (Mean) | OLP | 53.5 months | Fixed partial prosthetic |
| 2003 | Eposito et al. | clinical report | 2 female | 4 | 0% | 72/78 | OLP | 21 months | Over denture |
| 2008 | Gallego et al. | case report | 1 female | 2 | 100% | 81 | OLP | 36 months | Over denture |
| 2006 | Czerninski et al. |
case report | 1 female | 3 | 100% | 52 | OLP | 36 months | Fixed prosthetic |
| 2013 | Czerninski et al. |
retrospective controlled study | 11 female 3 male |
54 | 0% | 59.5 (Mean) | OLP | 63 months | Fixed partial prosthetic |
| 2000 | Esposito et al. | case report | 1 female | 2 | 100% | 69 | OLP | 32 months 60 months |
Over denture |
| 2005 | Oczakir et al. | case report | 1 female | 4 | 0% | 74 | OLP | 72 months | Fixed prosthetic |
| 2012 | Portela Tejedor | literature review in related to 3clinical cases | 2 female 1 male |
8 | n.a. | 53,59,59 | OLP | n.a. | Fixed prosthetic (third case awaiting rehabilitation) |
| 2013 | Altin et al. | Clinicl report | 1 female | 2 | n.a. | 70 | PV | 32 months | Over denture |
| 2010 | Ergun et al. | case report & literature review | 1 female | 6 | n.a. | 49 | SLE | 24 months | Fixed prosthetic |
3.1. Oral Lichen Planus
The literature search yielded 23 publications about OLP, of which 10 met the inclusion criteria of our systematic review. Most studies were single case reports or case series. A total of 60 patients with a mean age of 64.96 years and domination of females were reported in those studies (46 women and 14 men). Hence, 199 inserted implants were followed up for mean 42.40 months.
Just eight of the 10 articles could meet the inclusion criteria of meta-analysis. Two of them excluded because of data deficiency in survival rate and months of follow up. Table 2 shows the data of articles that met the inclusion criteria of our meta-analysis. The result of random effects analysis showed overall failure rates 22.0 (95% CI: 4.4 - 48.1).
| Variable for Studies | Authors Name & Date | |||||
| Variable for Total Number of Cases | Total Implants | |||||
| Variable for Number of Positive Cases | Number of Failures | |||||
| Study | Sample size | Proportion (%) | 95% Confidence Interval | Weight (%) | ||
| Fixed | Random | |||||
| Reichart 2006 | 10 | 0.000 | 0.000 to 30.850 | 7.69 | 13.96 | |
| Hernandez 2012 | 56 | 0.000 | 0.000 to 6.375 | 39.86 | 16.00 | |
| Eposito 2003 | 4 | 0.000 | 0.000 to 60.236 | 3.50 | 11.74 | |
| Gallego 2008 | 2 | 100.000 | 15.811 to 100.000 | 2.10 | 9.82 | |
| Czerninski 2006 | 3 | 100.000 | 29.240 to 100.000 | 2.80 | 10.94 | |
| Czerninski 2013 | 54 | 0.000 | 0.000 to 6.603 | 38.46 | 15.98 | |
| Esposito 2000 | 2 | 100.000 | 15.811 to 100.000 | 2.10 | 9.82 | |
| Oczakir 2005 | 4 | 0.000 | 0.000 to 60.236 | 3.50 | 11.74 | |
| Total (fixed effects) | 135 | 2.865 | 0.802 to 7.102 | 100.00 | 100.00 | |
| Total (random effects) | 135 | 22.029 | 4.376 to 48.097 | 100.00 | 100.00 | |
As the included articles were quite a few, we test them for heterogeneity. Table 3 proves the heterogeneity of data. So we report the random effect analysis that is more reliable than the fixed effect in these cases. Heterogeneity of the quality and data structure of included articles did not allow further comparative data analysis.
| Q | 54.3487 |
| DF | 7 |
| Significance level | P < 0.0001 |
| I2 (inconsistency) | 87.12% |
| 95% CI for I2 | 76.79 o 92.85 |
Results of the different studies, with 95% Confidence Interval (CI), and the pooled proportions with 95% CI are shown in Forest Plot (Fig. 2).
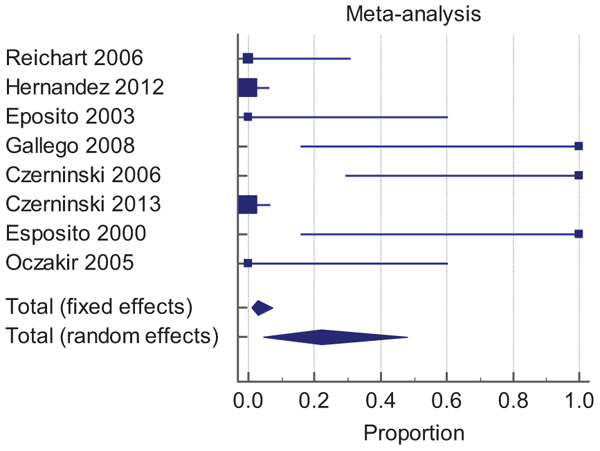
In this figure, the size of the markers represents the effects of the studies. The bigger square, the higher the weight. Moreover, diamonds represent the pooled effects. The location of the diamonds represents the estimated effect size and their widths reflect the precision of the estimate. The higher width, the less precision.
So the study of Hernandez (2012) with random weight 16.00% is the most effective study and studies of Gallego(2008) and Esposito (2000) with 9.82% random weight have the fewest effect in this meta-analysis.
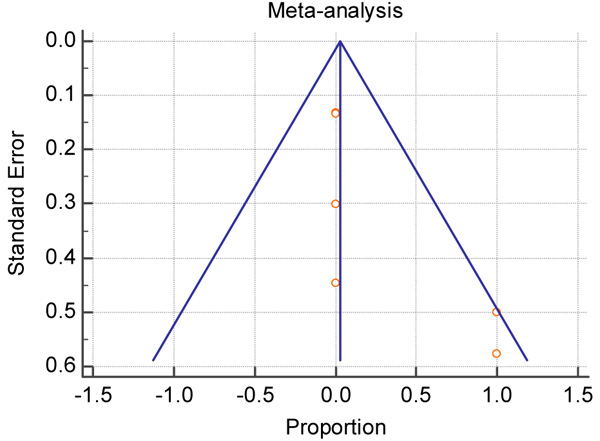
As I-square index is very large and statistically significant far from zero, and the funnel plot (Fig. 3) is asymmetry, therefore, the results of random effect are more reliable.
3.2.Pemphigoid
The literature search revealed no publications in this specific subject.
3.3. Pemphigus Vulgaris
The literature search yielded one publication about PV, which was the only clinical report that met the inclusion criteria. The authors reported a 70-year-old woman with two implants that were followed up for 32 months. The implant survival rate was not reported.
3.4. Systemic Lupus Erythematosus
The literature search revealed two publications, of which one of the studies met the inclusion criteria. The authors presented a 49-year-old woman and 24 months follow up of six inserted implants.
In SLE, PV, and pemphigoid we did not have enough articles to include them into our meta-analysis.
4. DISCUSSION
4.1. Oral Lichen Planus
Of the included articles about oral rehabilitation in oral mucocutaneous diseases, many of them described patients with OLP. In 2000, a case report about a 69-year-old woman with bruxism, poor bone quality, and erosive OLPwas published. That study stated that the severe immune response might be plaque induced but might also be related to OLP. The authors concluded that no definite correlation could be established between multiple implant failures and OLP because of the presence of different risk factors [45].
A clinical report in 2003 suggested that the use of overdenture with ball attachments reduced the incidence of erosive lesions. Although some unpublished clinical observations seem to contraindicate the use of implants [46-48], this article shows successful treatment in these patients who were treated with topical steroids, steroid rinse, and systemic corticosteroids through their care period [49].
In 2006, three cases were reported by Reichart and colleagues, which support the findings of the last research that was done by Esposito et al., in 2000. The authors suggest that implants may function in asymptomatic and even in the erosive type of OLP with no adverse effect. But to date, the total number of cases of the implant in these cases is small and also long-term follow-ups are limited [22].
In 2013, Czerninski et al., reported a retrospective study and revealed that there was no significant difference between patients with OLP with and without implant insertion. The authors supposed that low-dose topical steroids did not result in adrenal suppression and adverse systemic effect. Although in severe cases, patients received short courses systemic steroids. By the way, the ideas about the effect of local or systemic use of steroids on implant osseointegration are controversial and studies are limited [50].
In 2012, Hernandez and colleagues in their prospective controlled study inserted 56 implants for 18 patients. They stated that desquamative gingivitis should be examined carefully in follow-up periods because this condition is associated with peri-implant mucositis at a higher rate. Finally, they concluded that despite a small sample size and short follow-up, OLP was not associated with a high rate of implant failure [51].
In 2012, Petruzzi et al., demonstrated that there was not an absolute contraindication for implant rehabilitation and implants should be inserted in the remission phase of OLP with consideration of careful oral hygiene and frequent follow-up [52].
A narrative review in 2014 was published, which concluded that the extent and severity of underlying disease should be weighed before surgery. The researchers related the implant failure to the limited capacity of epithelium to adhere to the implant surface and they observed that peri-implant mucositis and peri-implantitis appeared more in patients with OLP. In the end, they suggested that OLP was not a reason for these failures while other risk factors such as parafunction, poor bone quality, and poor oral hygiene were considered as key causes [26].
A cross-sectional study done by Lopez-Jometand colleagues in 2014 suggests that implants do not influence the manifestations of OLP and OLP is not a risk factor for peri-implantitis. Also, the authors are in agreement with Czerninski and others, that implant rehabilitation does not influence the severity of OLP [21].
The last research that was done in 2016 is a systematic review by Reichart and co-workers who suggested that the spectrum of implant indications had been widened and included some mucocutaneous diseases. Also, they argued that despite the low prevalence of such diseases, many of them occur after 50 years of age when most of the affected patients are in need for prosthesis and implant supported ones could take a better action. Finally, the researchers concluded that the survival rate between the patients with or without mucosal diseases seemed to be comparable and there was no strict contraindication for implant insertion [44].
4.2. Pemphigoid
No article about dental implants in patients with pemphigoid met the inclusion criteria.
4.3. Pemphigus Vulgaris
There was just one study that addressed the clinical challenges associated with a dental implant in a patient with PV. This clinical report presented a 70-year-old woman who suffered from PV with a 32 months follow-up in 2013. The patient was followed up every 6 months and after 32 months just 0.9 mm peri-implant bone resorption was seen.
Although in this case, there was no real problem for implants, such patients may represent a risk for the clinical outcome of implants, which indicates an urgent need to do more studies [53].
4.4. Systemic Lupus Erythematosus
The only included article published in 2010 stressed that patients who took hydroxychloroquine showed no significant delay in healing process postoperatively. That report is in contrast with the side effect of immunosuppressive therapy, which extends the period of healing and decreases the possibility of infection. The authors concluded that not only there was no complication during either implant insertion or follow-up periods, but also they recommended dental implantation as a preferred approach in oral rehabilitation of these patients [54].
These articles revealed that there was no absolute contraindication for implant insertion in patients who suffer from mucocutaneous diseases; although they showed that there were some failures. But because of the presence of other risk factors, they cannot consider mucocutaneous diseases as the only cause of failures.
CONCLUSION
According to the meta-analysis and the results of the reviewed articles, implant failure rate in oral lichen planus is 22%. Due to the lack of adequate studies in PV, Pemphigoid, and SLE, a meta-analysis was only possible for Lichen planus.
Presently, there is no definite guideline for such patients; nevertheless, we should always consider that these patients are specific cases and need more attention in the first step of treatment and follow-ups.
Owing to a lack and heterogeneity of studies about dental implantations in patients with mucocutaneous diseases, the dilemma of implant insertion in these patients still remains and such results should be considered with caution. Doing more studies by considering different detailed risk factors such as type of drug used, duration of disease, oral hygiene, type of prosthesis, type of implants, and oral habits are required for definitive conclusions.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


