All published articles of this journal are available on ScienceDirect.
Shear Bond Strength of Two Types of Glass Ionomer to Bleached Dentin: Effect of Delayed Bonding and Antioxidant Agent
Abstract
Background:
Studies have shown a reduction in bond strength of composites and glass ionomer to bleached enamel and dentin. Several methods have been proposed to reverse compromised bond strength.
Objective:
The aim of this study was to evaluate the effect of delayed bonding and application of antioxidant agent on the bond strength of reinforced self-cured (Fuji IX) and light-cured glass ionomers (Fuji II LC) to bleached dentin.
Material:
Eighty extracted third molars were randomly divided into 8 groups. Buccal dentin surfaces received different treatments: Two control groups: no treatment + bonding Fuji IX or Fuji II LC. Two immediate bonding groups: bleaching + bonding Fuji IX or Fuji II LC. Two delayed bonding groups: bleaching + 7 days delay + bonding Fuji IX or Fuji II LC. Two sodium ascorbate application groups: Bleaching + application of 10% sodium ascorbate + bonding Fuji IX or Fuji II LC. All samples were tested for shear bond strength. Two-way analysis of variance (ANOVA) was used to compare the mean and standard deviations among groups, followed by the Tukey’s test for significant interaction.
Results:
No statistically significant difference was detected in shear bond strength of Fuji IX to bleached or normal dentin. Although a significant reduction was found shear bond strength values of Fuji II LC to bleached dentin, no significant difference was observed between no bleaching group and those treated with 10% sodium ascorbate or 7 days of delay in bonding for both types of glass ionomer.
Conclusion:
Bleaching had no significant effect on shear bond strength of Fuji IX to dentin; this type of GI can be used immediately after bleaching.
INTRODUCTION
One of the major dental problems affecting people of various ages in dentistry is tooth discoloration especially in endodontically treated teeth [1, 2]. White teeth are accepted as an important factor in beauty. Bleaching is a conservative, inexpensive, simple and fast treatment for discolored teeth [2, 3].
After whitening, the tooth colored resin restorations may not be harmonious with the surrounding bleached tooth structure, and they need to be replaced [4, 5]. In addition, in non-vital bleaching treatment, the bleaching agent is in direct contact with dentin, and access cavity must be restored consequently [6, 7].
Glass ionomer materials have unique qualities such as; the favorable adhesion to tooth structure, minimal effect on the pulp, suitable for areas that are difficult to isolate, high tissue tolerance and fluoride-releasing [8-10]. Due to these characteristics they have a wide range of applications as anterior proximal restoration, cervical restoration especially in areas with little or no enamel margins, and as a base material for composite restoration to reduce polymerization shrinkage and associated stresses in tooth structure as well as the tooth-restoration interface with subsequent problems of marginal leakage, recurrent caries and possible tooth fracture especially in endodontically treated teeth [11-13].
The original glass ionomer contains a fluroalminosillicate glass that is mixed with a polyalkenoic acid [11]. In order to improve their physical properties, and reduce their water sensitivity, future variation of glass ionomers occur by adding the water-soluble resin like hedroxyethyl methacrylate (HEMA) to produce resin-modified glass ionomer or light-cured glass ionomer [13, 14]. This novelty leads to superior beauty and polishing of these restorations immediately after the light curing of the resin [15].
By reducing the dimension of glass particle in the matrix of glass ionomer in materials such as Fuji IX (GC, Tokyo, Japan), reaction between the glass particle and polyacrilic acid is accelerated. This new generation of self-cured glass ionomer has been named as fast-setting, high-strength, or reinforced glass ionomers [13]. These materials have lower early moisture sensitivity and higher mechanical properties related to conventional materials. The manufacturers of Fuji IX suggest that this material is appropriate for Class I, II and V restorations in permanent and primary teeth [13].
Bleaching has adverse effect on the bond strength of restorative materials to dentin [6, 14]. Different theories have been suggested for such reduction in bond strength, including: calcium/phosphate (ca/p) reduction [10], presence of the bleaching gel remnants inside the dentinal tubules and within the collagen matrix [16, 17] residual of peroxide and free radicals of oxygen act as polymerization-inhibitor [17, 18]. Some techniques like removal of enamel surface layer [19], application of alcohol on bleached dental structure [20], the use of adhesive containing organic solvent [21] and delayed bonding(18) have been used to achieve reduced bond strength. Besides, some researches emphasize on neutralizing oxygen using different antioxidant agents [10, 17, 22] .
Sodium ascorbate (SA) as a biocompatible, inexpensive and available antioxidant agent, has been proven to facilitate the polymerization of bonding agent, and recover the bond strength of resin to dentin [10, 17, 22] Reduction of bond strength of glass ionomers to bleached dentin has been reported [18, 23]. In one study, the application of SA improved the bond strength of FUJI II LC to bleached dentin [8]. However to date, no information is available on the effect of delayed bonding and SA on the bond strength of FUJI IX to bleached dentin. Therefore, the purpose of this study was to evaluate the shear bond strength (SBS) of FUJI IX and FUJI II LC to bleached dentin; and the effect of SA and delayed bonding on their SBS to bleached dentin. The null hypothesis was that bleaching agent has no adverse effect on SBS of FUJI II LC and FUJI IX to dentin.
MATERIALS AND METHODOLOGY
Materials and their mode of application were summarized in Table 1.
Sample Preparation
Following the appropriate university human research ethics board consent; eighty non-carious sound human third molars not more than 3 months post extraction collected from the Dental Faculty of Tehran University of Medical Sciences were used in this research. The teeth were stored in 0.5% chloramines T at 4° C awaiting research initiation and used the teeth not more than 3 months post extraction. The tooth surfaces were first cleaned from debris and calculus using a universal curette (Deppeler, Rolle, Switzerland) and then polished with pumice powder, water and polishing rubber. The criteria for tooth selection were: no restoration, no crack, no hypoplasia, no hypocalsification and no history of any chemical application. Presence of crack in teeth was identified with the aid of a stereomicroscope at magnification of 30 x (Nikon, SMZ10; Tokyo, Japan). Each tooth was sectioned at 2 mm below the cementoenamel junction (CEJ) using a slow speed diamond saw (Isomet, Buehler Ltd, Lake Bluff, NY, USA) under abundant water spray. The teeth were horizontally embedded in auto-polymerized transparent acrylic resin (Acropars, Marlic Co, Tehran, Iran) blocks while the buccal surfaces of teeth were at the level of acrylic resin. Each buccal surface of the teeth was then grounded with 600 girt silicon carbide (SiC) wet abrasive paper on polishing machine (Malek Teb, Iran) to produce approximately a flat standard surface 1 mm deep into dentin .The blocks were then tested under a stereomicroscope (Nikon, SMZ10; Tokyo, Japan) to be certain that they were 1 mm deep in dentin. Samples were equally and randomly divided into 8 experimental groups (n=10) (Table 2).
| Material (manufacturer) | Mode of application |
|---|---|
| Fuji IX (GC Co,Tokyo,Japan) | GC conditioner was applied for 20 s, rinsed and dried for 10 s.1 level scoop of powder to 2 drops of liquid was dispensed onto the pad and mixed for 15–20 s. The mixture was transferred to the plastic mold |
| Fuji II LC(GC,Tokyo,Japan) | GC conditioner was applied for 20 s, rinsed and dried for 10 s.1 level scoop of powder to 2 drops of liquid was dispensed onto the pad and mixed for 15–20 s. The mixture was transferred to the plastic mold and light-cured for 40 s. |
| Opalecsence Xtra Boost (Ultradent Product,Inc,South Jordan,UT,USA) | The activator syringe was mixed with the bleaching agent 20 times, 1.0 mm thick layer was applied, agitated every 5 min, suctioned after 10 min, cleaned surfaces with water. This cycle was repeated twice every 3 days. |
| Sodium Ascorbate(powder)(Merck KGaA,Danrmatad, Germany) | The fresh sodium ascorbate (10%) solution was prepared by dissolving sodium ascorbate in distilled water at room temperature. The solution was applied for 20 min .Specimens were finally rinsed with distilled water for 10 min and dried with air. |
| Fuji IX | NC IX | Bonding Fuji IX |
| PC IX | Bleaching+ Bonding Fuji IX | |
| DB IX | One week delay+ Bleaching+ Bonding Fuji IX | |
| SA IX | SA+ Bleaching+ Bonding Fuji IX | |
| Fuji II LC | NC II | Bonding Fuji II LC |
| PC II | Bleaching+ Bonding Fuji II LC | |
| DB II | One week delay+ Bleaching+ Bonding Fuji II LC | |
| SA II | SA+ Bleaching+ Bonding Fuji II LC |
Bleaching
Whitening process was done with 38% hydrogen peroxide bleaching agent (Opalecsence Xtra Boost) based on the manufacturer’s instruction in all groups except NC II and NC IX (Table 1). The teeth were stored in incubator (100% humidity at 37ºC temperature) in bleaching time and between sessions. The negative control group samples were only reserved in incubator (100% humidity at 37°C temperature).
Antioxidant Agents
Following the final treatment part with whitening agent, SA solution was applied on specimen surface of groups SA IX and SA II for 20 minutes in incubator (100% humidity at 37°C temperature) (Table 1) . The sodium ascorbate crystals were dissolved by washing and plunging the samples in distilled water for 10 minutes.
Bonding Methods and Shear Bond Strength
The entire bonding procedures were done based on manufacturer’s instruction with one operator during the tests. 40 cylindrical samples (3.5 mm diameter × 4 mm height) of each kind of Glass- ionomers were attached to the dentin as follows:. The bonding site (3.5 mm circular area) was marked with a small piece of vinyl tape, wherein 3.5 mm-diameter hole had been prepared. 10% polyacrylic acid (GC Dentin Conditioner – GC Corporation, Tokyo, Japan) was applied to the limited surfaces for 20 seconds, rinsed with water for 10 seconds and air dried. Subsequently, the plastic mold (3.5 mm diameter × 4 mm height) was positioned and fixed on treated dentin surface and filled with the glass-ionomers (Fuji IX and Fuji II LC) . The Fuji II LC glass ionomer filling material (GC Corp, Tokyo, Japan) was blended (Table 1) and packed in two layers (2 mm height). Each layer was cured for 20 seconds with LED curing unit (Ivoclar Vivadent, Liechtenstein, Austria) with light intensity of 480 mW/cm2. In the Fuji IX groups, filling material was mixed as the explanation in Table 1 and packed into molds. The absolute setting of these samples occurred 6-minute after start of mixing. All samples were stored in incubator (100% humidity at 37°C) for one day prior to the shear bond strength test. The shear bond strength was measured by universal testing machine (Zwick, Ulm, Germany) at a crosshead speed of 0.5 mm/ min. Each sample was attached in a custom-made jig. The knife edge blade was positioned parallel to the surface at the junction of bonded to dentin samples. The shear bond strength was calculated in MPa. One operator evaluated the fracture surfaces with a stereomicroscope (Nikon, SMZ10; Tokyo, Japan) at 60 x magnification. Failure modes were classified like cohesive (if the fracture happened inside the glass ionomer or dentin), adhesive (if the fracture location was wholly inside the interface between the glass ionomer and dentin, mixed (if the fracture surpassed the interface into the glass ionomer or dentine).
RESULTS
Shear bond strength results (SBSs) are shown in Fig. (1). Results of one way ANOVA revealed significant differences in bond strength between Fuji II LC groups (P<0.05). However, no significant difference was detected in this respect between Fuji IX GP groups (P>0.05).
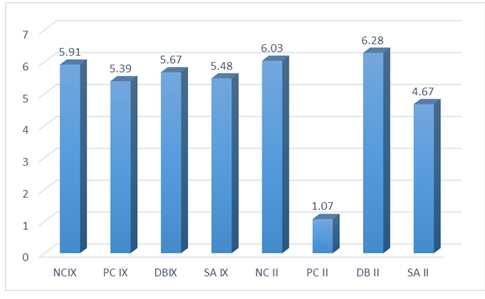
Results of POST HOC test demonstrated significant differences in bond strength between the (PC II) group with three other groups(p=0.00).
Also, significant differences were detected between positive control groups in Fuji IX GP and Fuji II LC. No significant difference was found between the two types of glass ionomers in other groups. Failure modes were mainly adhesive in all groups, except in the (PC II) group most of the samples failed before the bond strength test (Table 3).
DISCUSSION
Results of this study rejected part of the null hypothesis. Bleaching did not have negative effect on the bond strength of Fuji IX to dentin, while the bond strength of Fuji II Lc dramatically decreased.
Based on the theory of calcium/phosphate (Ca/P) reduction in bleached dentin [10, 24], it was expected that bleaching would reduce bond strength of both types of glass ionomers. However, bond strength of Fuji IX immediately after bleaching did not reduce. This finding may be related to the difference in curing and bonding mechanism of these two types of glass ionomers. In fast-setting chemical cure glass ionomers, the curing mechanism is the result of acid-base reactions between glass particles and polyacrylic acid molecules and formation of metallic salt bridges, and the bonding mechanism is due to chelating of polyacid molecules with calcium of dentin surface, also to a lesser extent, micromechanical locking of glass ionomers into dentinal surface irregularities [25]. This finding reveals that Ca/P reduction is not great enough to disrupt the ionic bond and the residual hydrogen peroxide and oxygen did not have a negative impact on curing mechanism and bonding of Fuji IX glass ionomer. This is not in accordance with the finding of Titley et al. revealed that bleaching of bovine dentin reduced bond strength of Fuji II glass ionomer [23]. They etched the dentin surface with phosphoric acid for 60s prior to bleaching. It seems that the cumulative effects of etching and bleaching on calcium removal might have been the reason of the reduced bond strength of conventional Fuji II glass ionomer.
| Group code | Adhesive | Mixed | Cohesive | Premature failure |
|---|---|---|---|---|
| NC IX | 8 | 1 | 1 | 0 |
| PC IX | 7 | 2 | 1 | 0 |
| DB IX | 9 | 1 | 0 | 0 |
| SA IX | 7 | 3 | 0 | 0 |
| NC II | 7 | 2 | 1 | 0 |
| PC II | 4 | 0 | 0 | 6 |
| DB II | 6 | 4 | 0 | 0 |
| SA II | 6 | 2 | 2 | 0 |
The curing mechanism of resin-modified glass ionomers is the combination of conventional glass ionomers and free radical methacrylate reaction. The methacrylate reaction starts faster than acid-base reaction [11]. In SEM analysis of dentin surfaces bonded with resin modified glass ionomer, resin tags were seen in dentinal tubules in glass ionomer-dentin interface indicating penetration of HEMA in the Fuji II LC into the collagen network [26-28].
Released oxygen after bleaching can interfere with [29, 30] or prevent resin polymerization [25, 29, 31-33] of resin-modified glass ionomers. It seems that the resin-modified glass ionomers will not cure near the dentin surface and bonding will not occur. In this study the immediately bonded resin- modified glass ionomer group failed mainly before the bond strength test, which supports this theory.
Based on the results of this study, the most probable role of bleaching in decreasing bond strength is through oxygen release from tooth surfaces. Therefore, to prevent this effect, the remaining oxygen should be removed from tooth structure [33].
In addition, some researchers have recommended a delay between bleaching time and restoration process [34]. This study revealed that one week delay in restoring the teeth after bleaching will increase the bond strength to the level of control group. This result is in agreement with other studies [18].
Although one week delay after bleaching is an efficient way to return bond strength of RMGI, in some situations it is a requisite to restore teeth immediately after bleaching to achieve an ideal seal especially in endodontically treated teeth [35].
The use of antioxidant agents to neutralize free radicals and reactive oxygen species has been recommended by a number of studies. Recent studies have demonstrated that 10% sodium ascorbate after bleaching can increase bond strength of composite resins to its initial level [32, 34]. The minimum recommended time for antioxidant agent to work effectively is one third of bleaching time [32, 33].
In this study, the level of bond strength after application of 10% sodium ascorbate solution for 20 min was similar to the levels of one week delay and control group. This procedure might eliminate the anticipated postponement for the restoration of bleached teeth [32].
In Fuji IX groups, no significant difference was seen in bond strength between the control group and the three other groups. This material can be used for tooth restorations in non-stress bearing areas and as a liner immediately after bleaching i.e. for access cavity filling in non-vital bleached teeth.
We evaluated the shear bond strength of glass ionomer to superficial bleached dentin without any aging. It should be pointed out that several variables exist in in vivo conditions that do not reflect in in vitro situation. Future studies should be developed to assess the stability of the bond strength of ionomer to dentin.
CONCLUSION
Within the limitation of this study, the following conclusions were drawn:
- Bleaching has no negative effect on bond strength of fast-setting, high-strength chemical cure glass ionomers such as Fuji IX to dentin and this restorative material can be used immediately after bleaching.
- Bleaching has negative effect on bond strength of resin-modified glass ionomers to dentin. In this regard, application of 10% sodium ascorbate or one week delay after bleaching increases bond strength.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This research has been supported by the Dental School of Tehran University of Medical Sciences, grant (grant number 89-02-69-10116).


