All published articles of this journal are available on ScienceDirect.
A One-year Follow-up Study of a Tapered Hydrophilic Implant Design Using Various Placement Protocols in the Maxilla
Abstract
Purpose:
To study the clinical/radiographic outcomes and stability of a tapered implant design with a hydrophilic surface when placed in the maxilla using various protocols and followed for one year.
Methods:
Ninety-seven consecutive patients treated as part of daily routine in two clinics with 163 tapered implants in healed sites, in extraction sockets and together with bone augmentation procedures in the maxilla were evaluated after one year in function. Individual healing periods varying from 0 to 6 months had been used. Insertion torque (IT) and resonance frequency analysis (RFA) measurements were made at baseline. Follow-up RFA registrations were made after 6 and 12 months of loading. The marginal bone levels were measured in intraoral radiographs from baseline and after 12 months. A reference group consisting of 163 consecutive straight maxillary implants was used for the comparison of baseline IT and RFA measurements.
Results:
Five implants failed before loading, giving an implant survival rate of 96.9% and a prosthesis survival rate of 99.4% after one year. The mean marginal bone loss after one year was 0.5 mm (SD 0.4). The mean IT was statistically significantly higher for tapered than for straight reference implants (41.3 ± 12.0 Ncm vs 33.6 ± 12.5 Ncm, p < 0.001). The tapered implants showed a statistically insignificantly higher mean ISQ value than the straight references implants (73.7 ± 6.4 ISQ vs 72.2 ± 8.0 ISQ, p=0.119). There was no correlation between IT and marginal bone loss. There was a correlation between IT and RFA measurements (p < 0.001).
Conclusion:
The tapered implant showed a high survival rate and minimal marginal bone loss after one year in function when using various protocols for placement. The tapered implant showed significantly higher insertion torque values than straight reference implants.
INTRODUCTION
Tooth replacement utilizing implant-supported prosthetic devices has been demonstrated to be a predictable treatment modality based on 50 years of clinical experience and long-term follow-up studies [1-3]. The present trend is to utilize one-stage and early/immediate loading protocols in order to speed up procedures and reduce patient discomfort [4]. In modern implant dentistry implants are also used in challenging situations due to compromised anatomy that in the past was considered as inappropriate for implant treatment. Several surgical techniques such as sinus floor elevation, split crest and guided bone regeneration (GBR) have found widespread use to compensate for insufficient bone volumes [5-7]. Placement of implants in fresh extraction sockets becomes ever more practiced in daily routine [8]. Dental implant producers are trying to improve outcomes by modifying the macroscopic design and the surface of the implants, thus increasing primary stability and osteoconductive properties, which has proven to be of determinant importance for osseointegration [9]. With regard to macroscopic design, studies have shown improved primary stability for implants with a tapered body compared to a parallel-walled design [10-12]. With regard to implant surface it is evident that a certain degree of micro-roughness (moderate roughness) results in a stronger bone tissue response than to a smooth-surfaced (minimally rough) implant [13-15]. The majority of modern implants have a moderately rough surface as produced by blasting, etching and anodic oxidation or a combination of these techniques [9].
Clinical studies on the Neoss implant design (Neoss Straight) with a minimally rough blasted surface (Bimodal) have demonstrated high survival rates and minimal marginal bone loss with two-stage protocols and other more challenging procedures after 1 to 5 years of follow-up [16, 17]. However, this surface has shown more prone to failure in early loading [18] and in GBR cases [17] compared to the newer hydrophilic and moderately rough surface (Proactive) [18, 19]. In addition, a tapered implant (Neoss Tapered) with the moderately rough surface has been developed and launched on the market. This design has been demonstrated to increase primary stability in comparison with the original design and particularly in soft bone densities in an in vitro investigation [20], which is in line with previous studies [10-12]. It can be speculated that the combination of a tapered design and a hydrophilic moderately rough surface may facilitate placement and osseointegration of implants and particularly in challenging situations. However, no clinical studies on this implant design have been presented.
The aim of the present follow-up clinical study was to evaluate the clinical performance of the Neoss Tapered implant during one year of loading.
MATERIALS AND METHODS
Patients
Ninety-seven consecutive patients (56 females and 41 males, mean age of 55.6 years, range 30 to 86 years) that had been treated with Neoss Tapered implants (Neoss Ltd. Harrogate, UK) in the maxilla in two clinics as part of daily routine were evaluated after one year of loading (Table 1). Eighteen months passed between the days of the first and the last implant placement in this study.
| Patients | Implants | ||||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Study center | 1 2 |
22 75 |
22.7 77.3 |
39 124 |
23.9 76.1 |
| Gender | Female Male |
56 41 |
57.7 42.3 |
95 68 |
58.3 41.7 |
| Smoker | No Yes Previous smoker |
72 21 4 |
74.2 21.6 4.1 |
114 40 9 |
69.9 24.5 5.5 |
| Bruxism | No Heavy bruxism |
78 19 |
80.4 19.6 |
133 30 |
81.6 18.4 |
| Periodontal problems | No Moderate Severe |
52 30 15 |
53.6 30.9 15.5 |
80 48 35 |
49.1 29.4 21.5 |
| Total | 97 | 163 | |||
Treatment planning was made based on clinical and radiographic examinations such as intraoral radiographs, orthopantomograms (OPGs) and in some cases computed tomography (CT) scans. All patients had given their written consent to the treatment plan and follow-up according to the routine procedures at the centres. The study was made in accordance with the World Medical Association Declaration of Helsinki. All patients had been treated and followed up according to the normal routines of the two clinics.
Surgery
No surgery was performed in the presence of acute intraoral infections. Chronic periodontitis, smoking and bruxism were considered to be the risk factors but not absolute contraindications (Table 1).
Surgery was performed under antibiotic prophylaxis with 2 gr of amoxicillin (Pensa Pharma, Milano, Italy) in local anesthesia (Mepivacaina/Adrenalina, Scandonest 2%, Septodont, France). In addition, 2 x 1 gr of amoxicillin was prescribed for 6 days after surgery

A total of 163 Neoss Tapered implants with Proactive surface implants (Neoss Ltd., Harrogate, UK) in lengths from 9 to 15mm and in diameters from 3.5 to 5.0 mm had been placed (Table 2) Fig. (1a). All implants were positioned in the maxillary arch.
| Diameter (mm) | |||||||
|---|---|---|---|---|---|---|---|
| 3.5 | 4.0 | 4.5 | 5.0 | 5.5 | Total | ||
| Length (mm) | 9 | 1 | 1 | 0 | 2 | 0 | 4 |
| 11 | 13 | 25 | 8 | 11 | 1 | 58 | |
| 13 | 17 | 42 | 16 | 9 | 0 | 84 | |
| 15 | 2 | 13 | 2 | 0 | 0 | 17 | |
| Total | 33 | 81 | 26 | 22 | 1 | 163 | |
Eighty-eight (88) implants had need for compensation of reduced bone volume or incomplete bone-implant contact. For 30 fixtures autogenous bone harvested from the drills at same surgical site or by a bone scraper (Micross, Meta, Reggio Emilia, Italy)was deposited on the implant surface without coverage with membranes. Bone substitutes (GenOs, Osteobiol, Turin, Italy) mixed with autogenous bone or not were applied to 58 implants of which 28 were covered with an additional resorbable membrane.
Sinus floor elevation using lateral approach and bone substitute material (Osteobiol, Turin, Italy) was performed for nine implants, of which four were positioned after six months of healing and five simultaneously with the elevation surgery. In addition, five implants were placed with a trans-crestal approach using osteotomes.
In six cases, a split-crest technique with piezo-surgical devices and/or hand driven chisels to augment the horizontal dimension of the bone wall to be able to place ten implants were used.
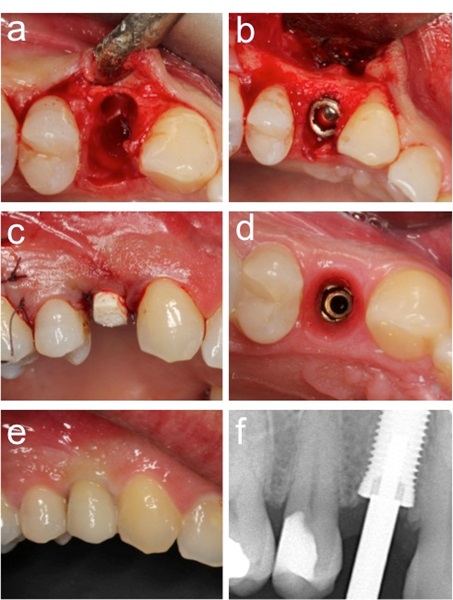
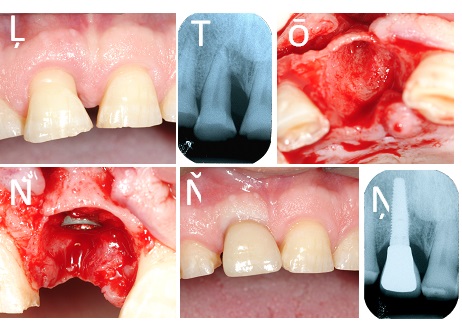
A total of 57 implants were placed in fresh extraction sockets, 11 in partially healed sockets and 95 in healed sites. In those cases in which the gap between bone and implant surface was small (<1.5 mm) no grafting material was used to fill this space. When the distance between implant surface and bone was 2.0 mm or more a bone substitute was utilized Osteobiol, Turin, Italy) (Fig. 2a and b, Fig. 3a and d). Under those circumstances, which did not allow a perfect primary wound closure around the healing abutment, a resorbable membrane (Evolution, Osteobiol, Turin-Italy) was applied to cover the material inserted in the implant/bone space.
A one-stage procedure using 2 or 5 mm PEEK healing abutments (Neoss Ltd, Harrogate, UK) was used for 124 implants (Fig. 2c and d) and 24 implants were inserted following a two-stage protocol. Second surgery was performed after two to five months of healing. Immediate loading with a temporary device was done on 15 implants.
During surgery, peak insertion torque was registered up to 50 Ncm with the torque control device of the drive unit W&H Implantmed (W&H Dentalwerk, Bùrmoos, Austria) in both clinics. When higher forces were needed, a manual wrench inserter was used and IT was registered as >50Ncm. Implant stability was measured with resonance frequency analysis (RFA) (Osstell ISQ, Osstell AB, Gothenburg, Sweden) in implant stability quotient (ISQ)units. Ortho-radial intraoral radiographs were taken at baseline and after 6 months and one year. Bone quality and quantity were registered using the Lekholm & Zarb index [21].
Prosthetics
The implants were loaded after different healing periods based on primary stability, risk factors inherent to surgical procedures as GBR or fresh socket placement and patients’ needs, varying from immediate loading to delayed loading after 6 months (Table 3). The implants supported 14 full arch bridges, 34 partial bridges, one over-denture on a bar support and 48 single teeth replacements. Screw-retained prosthetics were preferred and cemented constructions only used for angulated abutments on single tooth replacements. All but one of the full arch bridges were supported by a mixture of Neoss Tapered and Straight implants that were placed previous to the beginning of the present study. Those straight implants are not included in this study and this explains why such a low number (49) of Tapered implants were involved in the support of full-arch prosthetic devices. Only one full arch was applied to 4 implants, the others had 6 fixtures to sustain them.
| Total | ||
|---|---|---|
| n | % | |
| Immediate load | 15 | 9.2 |
| One-stage | 124 | 76.1 |
| Two-stage | 24 | 14.7 |
| Total | 163 | |
The majority of implants (157) were initially loaded with an acrylic provisional device of which 15 were loaded within 48 hrs after surgery. Four implants showing excellent stability and stable surrounding soft tissues were loaded directly with a single ceramic definitive crown. Definitive prosthetics on single crowns and partial bridges were all performed in ceramics on a noble metal-, disilicate- or zirconium basis (NeoLink, Neoss Ltd, Harrogate, UK) (Figs. 2e and 3e). Thirteen out of fourteen full arch bridges were using acrylic teeth supported by a CAD/CAM milled titanium framework. One arch was produced in CAD/CAM milled zirkonium layered with feldspatic ceramics.
Reference Group
A reference group of 78 patients previously treated with 163 Straight Neoss (Neoss Ltd., Harrogate, UK) Fig. (1b) in the maxilla in one of the centres was used for comparisons with the Tapered design with regard to insertion torque (IT) and resonance frequency analysis (RFA) measurements.
Follow-up
The patients had been examined after 6 and 12 months in function when the prosthetic constructions were removed except for cement-retained ones or when patient’s consent lacked. At this time implant stability was registered by means of RFA measurements in addition to the baseline registrations.
Intraoral radiographs were taken at baseline (abutment surgery or at prosthetic loading) and after 12 months of loading (Figs. 2f and 3f). The distance from the coronal platform to the first bone contact was measured on mesial and distal aspects using ImageJ software (NIH, Washington, US). A mean value was calculated for each implant and time point.
An implant was defined a failure as it had to be removed and a survival if clinically stable and supporting the prosthesis without causing discomfort to the patient.
Statistics
The statistical analyses were made with the SPSS Statistics 17.0. software (SPSS Inc., Chicago, IL, USA).
For the sub-group analysis on bone remodeling, Spearman Rank correlation was used for ordered sub-groups, Kruskal-Wallis test with post-hoc Bonferroni corrected pair-wise Mann-Whitney U tests were used for multiple unordered sub-groups, and Mann-Whitney U test was used in cases of two sub-groups.
The Mann-Whitney U test was used to identify differences in implant stability between straight and tapered implants. Friedman’s test with post-hoc Bonferroni corrected Wilcoxon tests were used to identify differences in implant stability over time.
The Pearson correlation test was used to identify correlations between insertion torque, ISQ values and bone loss. Significance level p<0.05 was used for all tests.
RESULTS
Clinical Observations
Five implants in three patients failed to integrate before functional loading giving a survival rate of 96.9% after one year of loading (Table 4). All five had high insertion torque and showed ISQ values above 70 (Table 5). Four of the failing implants were placed in fresh extraction sockets with a one-stage approach or immediate loading protocol. Three of these implants had been placed without CT-scans and with minimal flap elevation. When during replacement surgery a more extended flap was raised it became evident that apical bone volume had been overestimated. The fifth implant was lost due to infection of endodontic origin on an adjacent tooth. Endodontic treatment was done to eliminate the infection and the patient is waiting for second surgery to replace the implant lost. Three of the failing fresh socket implants were replaced in the same site after healing of the implant socket using inferior diameters of the implants. For one failing implant that was one out of three inserted simultaneously, the decision was made not to replace it and to restore function on the two surviving implants.
| Interval | Implants | Failed | Withdrawn | CSR |
|---|---|---|---|---|
| Insertion to 6 months | 163 | 5 | 0 | 96.9% |
| 6 months to 12 months | 158 | 0 | 9 | 96.9% |
| 12 months | 149 | - | - | 96.9% |
| Center | Pat. ID | Gender | Bruxer / Smoker / Perio | Pos. | Dimensions |
ISQ/ IT |
Site | GBR | Loading |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 118 | Female | No / No / Severe | 24 | 4.0 x 13 mm | 75/40 | IES | AB | Immediate |
| 2 | 203 | Female | No / No / No | 11 | 4.5 x 13 mm | 75/25 | IES | - | OS, not loaded |
| 21 | 4.5 x 13 mm | 76/25 | IES | - | OS, not loaded | ||||
| 2 | 237 | Male | No / No / No | 14 | 4.0 x 13 mm | 76/50 | HS | - | OS, not loaded |
| 2 | 250 | Female | No / Yes / Severe | 15 | 5.0 x 11 mm | 81/50 | IES | - | OS, not loaded |
From a prosthetic point of view, 76 of 77 constructions were loaded for one year, giving a prosthetic survival rate of 99.4%.
Marginal Bone Loss
Paired intraoral radiographs were obtained for 143 implants at baseline and after one year of follow-up. At baseline the average marginal bone level was 0.2 ± 0.3 mm and 0.7 ± 0.4 mm after one year (Table 6). The average bone loss was calculated to be 0.5 ± 0.4 mm (Table 7).
| Baseline | 12 months | |||
|---|---|---|---|---|
|
Mean (mm) S.D. n Range (mm) |
0.22 0.33 143 0.00 – 1.53 |
0.73 0.39 143 0.0 – 1.87 |
||
| Distribution | n | % | n | % |
| 0 mm | 97 | 67.8 | 13 | 9.1 |
| 0.5 mm | 36 | 25.2 | 63 | 44.1 |
| 1.0 mm | 7 | 4.9 | 54 | 37.8 |
| 1.5 mm | 3 | 2.1 | 11 | 7.7 |
| 2.0 mm | 0 | 0 | 2 | 1.4 |
| Baseline to 12 months | ||
|---|---|---|
|
Mean (mm) S.D. n Range (mm) |
-0.52 0.41 143 -1.56 – 0.48 |
|
| Distribution | n | % |
| +0.5 mm | 6 | 4.2 |
| 0 mm | 25 | 17.5 |
| -0.5 mm | 72 | 50.3 |
| -1.0 mm | 35 | 24.5 |
| -1.5 mm | 5 | 3.5 |
Frequency distribution showed bone loss over 1 mm for five implants and no implant showed more than two mm of bone loss (Table 7).
No significant differences could be found for average bone loss when comparing implants placed in fresh extraction sockets or using split crest bone augmentation and implants inserted in healed bone sites (Table 8). Neither was there a different response of the marginal bone when applying immediate loading protocol confronted to non-immediate loading (Table 8). There was no correlation between bone loss and insertion torque (Table 9).
| Procedure |
BL baseline (mm ± SD) |
BL 1 year (mm ± SD) |
Bone loss (mm ± SD) |
No |
|---|---|---|---|---|
| Extraction socket | 0.2 ± 0.2 | 0.6 ± 0.3 | 0.5 ± 0.4 | 58 |
| Non-extraction socket | 0.2 ± 0.4† | 0.8 ± 0.4† | 0.6 ± 0.4† | 85 |
| Immediate load | 0.3 ± 0.3 | 0.9 ± 0.4 | 0.6 ± 0.3 | 14 |
| Non-immediate load | 0.2 ± 0.3† | 0.7 ± 0.4† | 0.5 ± 0.4† | 129 |
| Split crest | 0.3 ± 0.6 | 0.7 ± 0.4 | 0.3 ± 0.5 | 10 |
| Non-split crest | 0.2 ± 0.3† | 0.7 ± 0.4† | 0.5 ± 0.4† | 133 |
|
Insertion torque (Ncm) |
Stability (ISQ ± SD)*** |
Bone loss (mm ± SD)† | No |
|---|---|---|---|
| 50 – | 76.2 ± 5.3 | 0.5 ± 0.4 | 94 |
| 40 – 45 | 72.3 ± 5.3 | 0.6 ± 0.4 | 21 |
| 30 – 35 | 70.0 ± 6.7 | 0.5 ± 0.3 | 31 |
| – 30 | 68.1 ± 6.2 | 0.5 ± 0.4 | 15 |
Implant Stability
The average IT at implant placement was 41.3 ± 12.0 Ncm for the tapered implants, which was significantly higher than for straight control implants (33.6 ± 12.5 Ncm)(p<0.001) (Table 10).
| Tapered | Straight | |||
|---|---|---|---|---|
|
Mean S.D. n |
41.3 12.0 163 |
33.6 12.5 138 |
||
| n | % | n | % | |
| < 10 | 2 | 1.2 | 0 | 0 |
| 10 – 19 | 4 | 2.5 | 6 | 4.3 |
| 20 – 29 | 11 | 6.7 | 34 | 24.6 |
| 30 – 39 | 31 | 19.0 | 41 | 29.7 |
| 40 – 49 | 21 | 12.9 | 18 | 13.0 |
| 50 – | 94 | 57.7 | 39 | 28.3 |
RFA showed an average ISQ value of 73.7 ± 6.4 at placement, 75.0 ± 4.5 ISQ after 6 months and 77.0 + 4.1 ISQ after a year of loading, increasing in a significant way over time (p=0.001). The tapered implants showed a higher average ISQ value than the straight control implants at placement, 73.7 ± 6.4 ISQ vs 72.2 ± 8.0 ISQ (Table 11). However, the difference was not statistically significant (p=0.119)
| Tapered | Straight | |||
|---|---|---|---|---|
|
Mean S.D. n Range |
73.7 6.4 161 53 - 84 |
72.2 8.0 163 30 - 85 |
||
| n | % | n | % | |
| 30 – 39 | 0 | 0 | 1 | 0.6 |
| 40 – 49 | 0 | 0 | 2 | 1.2 |
| 50 – 59 | 6 | 3.7 | 10 | 6.1 |
| 60 – 69 | 26 | 16.1 | 25 | 15.3 |
| 70 – 79 | 98 | 60.9 | 98 | 60.1 |
| 80 – 89 | 31 | 19.3 | 27 | 16.6 |
There was a statistically significant correlation between IT and RFA measurements at baseline (Table 9).
DISCUSSION
The reason for only reporting on the outcome of maxillary tapered implants in the present study is that implant treatment of the upper jaw is regarded as more challenging than of the lower jaw [22]. This is partly because of surgical challenges due to resorption patterns, presence of maxillary sinuses and nose cavity and the more frequent occurrence of soft bone compared to the mandible. The implant surgery in the present study was performed in two private general dental clinics, both focusing on periodontics and implant–supported oral rehabilitation. The same operator provided the patients with implants and prostheses. The high cumulative survival rate (96.9%) and the nearly complete success of the prosthetic outcome (99.4%) indicates that this type of fixture is predictably performing in standard implant situations as well as with more challenging protocols. The survival rate and average bone loss of 0.5 ± 0.4 mm encountered does not deViate from the results of previous studies on Neoss Straight implants [16-19, 23] neither from other implant systems [24]. An important number of implants was placed in fresh extraction sockets or when utilizing various bone augmentation protocols as sinus floor elevation, split crest and GBR showing slightly, though not significantly less bone loss as compared to healed implant sites.
Primary stability as expressed by insertion torque (IT) was significantly higher for Neoss Tapered implants as compared to the reference Neoss Straight implants from another patient group. The implant surface was the same for both designs, which indicates that the differences can be ascribed to differences in implant geometry as also demonstrated in previous studies [10-12, 20]. When comparing RFA measurements, statistically insignificantly higher ISQ values were obtained for the tapered implants, which is in line with a previous in vitro study comparing the two implant designs where only a subtle difference was seen for ISQ values in spite of a marked difference in IT [20]. However, there was a correlation between IT and ISQ, which is in line with other studies [25, 26].
The tapered implant showed similar or higher baseline ISQ values than reported for other implant types placed in maxillary bone [27, 28]. However, due to that the ISQ transducers are different for different implant system, it is difficult to make direct comparisons. Moreover, factors such as placement depth also influence the ISQ values [29, 30]. It is the authors’ understanding that IT gives information about the tightness of the bone/implant contact, whereas RFA expresses rigidness of a more extended bone/implant complex. The overall maxillary bone structure will not be altered by differences between straight and tapered implant osteotomies, although the tapered implant will probably result an increased local compression of the bone and tighter fixation. It is possible that this is not picked up by the vibrations induced by the RFA device as these will probably be absorbed in a similar way by the bony anatomy independent of the implant geometry.
Monitoring of ISQ during healing and loading reveals precious information about the stadium and quality of osseointegration and about the capability of the bone to absorb functional loading forces transmitted to it through the fixture [30, 31] and is a standard procedure for all implants positioned in the offices involved in this study. Neither high IT nor high ISQ do guarantee implant survival, as low values do not necessarily predict failure [32]. However, torque and stability measurements combined with operator experience are indispensable for decision making on subjects as one- or two-stage surgery and time of loading [30]. Moreover, studies have shown that continuous monitoring of ISQ values during immediate loading procedures is useful to identifying and avoiding implant failure [33, 34].
Concerns have been expressed that too high IT may induce pressure necrosis and marginal bone resorption [35]. In the present study, no correlation between IT and marginal bone loss after one year could be seen, which is in line with the conclusions of a recent meta-analysis of the literature including studies with reported ITs from < 25 to 176 Ncm [35]. Interestingly, our data showed that also low IT resulted in ISQ levels that are considered to indicate sufficient stability, even for early loading protocols [30, 36].
The tapered design showed high primary stability, also in relatively challenging anatomical and surgical conditions such as split crest- and GBR procedures or placement in fresh extraction sockets. This defines this fixture design eligible when compromised primary stability is to be expected due to reduced bone volume or presence of gaps between bone and implant surface after placement in sockets. Increased stability favours also predictability of immediate- or early loading protocols. The fact that all implants that had an extremely low IT and very low ISQ values at baseline because of split crest, sinus floor elevation, GBR-procedures or combinations of those led to complete integration confirms the biocompatibility and osteoconductivity of the ProActive surface as reported by other authors [37]. In all those cases RFA monitoring revealed increasing ISQ values from baseline to the 6 and 12 month follow-up registrations, which indicate a favourable bone tissue response to the implants. Four of five failures were due to other reasons than poor healing, i.e. operator mistake and infection.
CONCLUSION
It is concluded that the tapered implant design performs well when used in daily routine in the maxilla in healed sites, in fresh extractions sockets and in combination with various bone augmentation procedures. However, long-term clinical studies are needed to further evaluate this implant.
CONFLICT OF INTEREST
The authors did not receive any direct financial support of the study. However, Dr. Herman Sahlin is an employee of Neoss Ltd and helped with compilation and statistical analyses of the data.
ACKNOWLEDGEMENTS
Declared none.


