All published articles of this journal are available on ScienceDirect.
Promotion of Nickel (Ni) Allergy by Anamnestic Sensitization with a Bacterial Component, Lipopolysaccharide (LPS), in Mice
Abstract
Background/Objective:
Lipopolysaccharides (LPS) promote allergic responses to nickel (Ni) both in the sensitization and elicitation steps. In this study, we examine the effect of pre-sensitization to LPS on the occurrence of Ni allergy using a mouse model.
Method:
A 100 mg of LPS was injected into C57BL/6J mice intraperitoneally (ip). Three weeks later, the mice were subsequently injected with 0.3 μ moles of nickel dichloride (NiCl2) and 100 μg of CpG-DNA, which acted as an adjuvant. The mice were repeatedly immunized with the 0.3 μg of nickel sulfate (NiSO4), along with 300 μl of the adjuvant, Inject Alum (Pierce, USA). Then we examined the producing capabilities of T helper type 1 (Th1) and 2 (Th2) cytokines (interferon-gamma- (IFN)-γ and interleukin (IL)-10, respectively) from anti CD3 antibody-stimulated spleen cells.
Results:
Pre-treatment with LPS, followed by repeated challenges with Ni2+ and adjuvants significantly enhanced the IFN-γ-producing capability of spleen cells (n=5, p<0.01); however, that could not enhance the capability of spleen cells by a single challenge with Ni2+ and adjuvants (n=5). In contrast, without LPS treatment, single or even repeated challenges by Ni2+ could not enhance the IFN-γ-producing capability. On the other hand, the IL-10-producing capability of spleen cells was not enhanced even by LPS and repeated challenges with Ni2+ and adjuvants.
Conclusion:
The solitary pre-sensitization to LPS is essential for the onset of Ni allergy by shifting the Th1/Th2 immune balance toward a Th1 dominant.
INTRODUCTION
Metal allergies caused by exposure to sensitizing metals including nickel (Ni), cobalt (Co), chromium (Cr), palladium (Pd), and gold (Au) are well known [1]. Among them, Ni is the most common cause of metal-induced allergies in prosthodontic treatment [2, 3]. Allergic reactions induced by metals are, in general, classified as Type IV [1]. Based on cytokine production, two murine CD4 cell subsets, Th1 and Th2, are defined [4]. Th1 cells produce IL-2 and IFN-γ, while Th2 cells are characterized by their ability to produce IL-4 and IL-10 [5]. Th1/Th2 imbalance has been observed in acute or chronic infections, cancer, asthma, and other allergies [6-10]. The type IV allergy, delayed type hypersensitivity (DTH), is mainly regulated by Th1 cytokines [11].
However, the action mechanisms of Ni are pleiotropic. Ni exposure can also elicit Type I hypersensitivity [12] mediated by Th2 cells. Ni hypersensitivity is also evoked by hapten-mediated allergic reactions [13], and at the other extreme, Ni can directly bind to Toll-like receptor (TLR) 4 to sufficiently induce nickel sensitization [14]. Unlike typical ACD, the murine equivalent model virtually depends on cytotoxic T cells [15]. Interestingly, the Ni allergy can occur even in T-cell-deficient nude mice [16].
In addition to Ni, the bacterial environment is an important factor in the development of metal allergies [16]. LPS, the component of the outer membrane of Gram-negative bacteria, promotes allergic responses of Ni at both the sensitization and elicitation steps [17]. However, in these experiments, the effects of simultaneously injecting LPS as an adjuvant with Ni may not recreate the essential conditions associated with the anamnestic history of inflammation adjoining periodontitis.
In order to examine the pre-existing condition of LPS upon Ni allergy, we investigated the effects of anamnestic pre-treatment of LPS by evaluating the cytokine-producing capability of stimulated spleen cells from immunized mice.
MATERIALS AND METHODS
Animals
C57BL/6J mice (4 weeks old males) were purchased from SLC (JAPAN), and then acclimatized in our animal facility for 2 weeks.
Sensitization to Ni
At 6 weeks old, the mice's intra peritoneal cavities (IP) were injected with 100 µg of LPS (L8274, Sigma-Aldrich, USA) in a 200 µl physiological phosphate buffer saline (PBS). After the 3rd week, the mice (9 weeks old) were primarily immunized IP with the nickel (Ni) ion solution containing 0.3 µmoles of nickel NiCl2 and a 100 µg adjuvant of CpG-DNA (5’-TCC ATG ACG TTC CTG ACG TT-3’) [18, 19] in 600 µl of PBS. After 3 more weeks, the mice 12 weeks old were repeatedly immunized IP with the Ni ion solution containing 0.3 µg of nickel sulfate (NiSO4) in a 300 µg adjuvant of Inject Alum (Pierce, USA) in a 300 µl of PBS. Last, after an additional 3 weeks, spleen cells were extracted from the mice, now 15-weeks old, to determine their cytokine-producing capabilities.
Cytokine-producing Capabilities of Spleen Cells
We previously described the method for determination of cytokine-producing capabilities of murine spleen cells [20]. Briefly, 10 µg/ml of anti-CD3ε monoclonal antibody (mAb)(145-2C11) [21] in phosphate buffered saline (PBS) was immobilized (100 μl/well) on a 96-well tissue culture plate (3599, Corning, NY) at 4 oC for 4 hours. In order to remove red blood cells, isolated spleen cells were treated with 10 mM Tris-HCl (pH7.3) containing 140 mM ammonium chloride and 1 mM Na2EDTA. Before the supernatants were harvested, the cells (4 x 105 /well) were cultivated on the 96-well tissue culture plate coated with anti-CD3ε mAb for 48 hours under 5% CO2, in 200 µl of RPMI-1640 (R8758, Sigma-Aldrich, MO) containing 50 μM 2-mercaptoethanol, 100 units/ml penicillin, 100 µg/ml streptomycin, 250 ng/ml amphotericin B, and supplemented with a 10% fetal calf serum (Invitorogen, CA).
ELISA to Determine Concentrations of Produced Cytokines
Concentrations of IFN-γ and interleukin-10 (IL-10) produced by the spleen cells were determined by enzyme-linked immunosorbent assay (ELISA) with BD OptiEIA Set (Phermingen, CA) as described [20].
Statistics
All analyses were performed using Stat View Ver 5.0 software (SAS, NC). The data are expressed as means ± standard deviation (SD). Student’s t-test was applied to determine the significance of differences between the two groups. P values less than 0.01 were considered to be statistically significant.
RESULTS
Effects of Anamnestic Treatment with LPS on IFN-γ-producing Capability
We examined the effects of anamnestic pre-treatment with LPS on the induction of Ni-allergy. After 3 weeks of either LPS injection or a control injection of PBS, mice (6 weeks old) were immunized with the NiCl2 or with an adjuvant alone. After 3 more weeks, the Th1 cytokine IFN-γ-producing capability of anti-CD3ε-stimulated spleen cells was examined (Fig. 1a and Table 1). The IFN-γ-producing capability was significantly more attenuated in the NiCl2-injected mice than in the control group, regardless of LPS pre-treatments. The same series of mice were repeatedly immunized with NiSO4. After three more weeks, the induced IFN-γ-production of spleen cells was significantly higher in the mice which were pre-treated with LPS and injected twice with Ni, than in those sensitized with a single Ni injection. By contrast, without LPS treatment, the IFN-γ-producing capability was somewhat attenuated after the repeated Ni injections (Fig. 1b and Table 1).
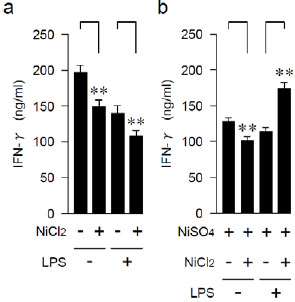
| LPS | NiCl2 | NiSO4 | #Mean | ± | SD | *P< |
|---|---|---|---|---|---|---|
| - | - | 201.8 | ± | 4.9 | 0.0001 | |
| - | + | 152.7 | ± | 5.9 | ||
| + | - | 142.9 | ± | 4.5 | 0.0001 | |
| + | + | 109.8 | ± | 2.8 | ||
| - | - | + | 126.6 | ± | 4.5 | 0.01 |
| - | + | + | 108.6 | ± | 2.3 | |
| + | - | + | 124.4 | ± | 4.4 | 0.001 |
| + | + | + | 183.0 | ± | 8.4 |
*Student’s t-test, NiCl2 innoculated (+) vs. uninnoculated (-).
Effects of Anamnestic Treatment with LPS on IL-10-producing Capability
Next, we examined the production of a Th2 cytokine, IL-10, in the same series of experiments. The IL-10-producing capability in LPS-untreated mice was attenuated by repeating the Ni injection (Fig. 2a and Table 2). In LPS-pretreated mice, the producing capability of IL-10 was unaffected even by repeating Ni injections (Fig. 2b and Table 2).
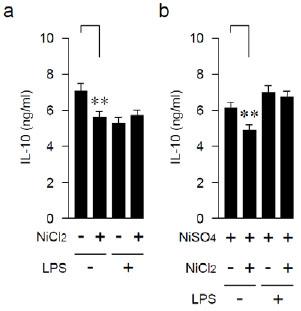
| LPS | NiCl2 | NiSO4 | #Mean | ± | SD | *P < |
|---|---|---|---|---|---|---|
| - | - | 7.29 | ± | 0.15 | 0.01 | |
| - | + | 5.57 | ± | 0.11 | ||
| + | - | 5.07 | ± | 0.10 | 0.06 | |
| + | + | 5.83 | ± | 0.12 | ||
| - | - | + | 6.28 | ± | 0.34 | 0.01 |
| - | + | + | 4.92 | ± | 0.23 | |
| + | - | + | 6.95 | ± | 0.19 | 0.17 |
| + | + | + | 6.73 | ± | 0.15 |
*Student’s t-test, NiCl2 innoculated (+) vs. uninnoculated (-).
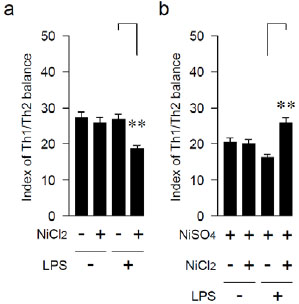
Effects of LPS and Ni on the Ratio of Th1/ Th2 Cytokines
An index of the Th1/ Th2 balance was calculated by dividing the level of the IFN-γ-producing capability with the level of the IL-10-producing capability. The index of the Th1/Th2 balances of mice with the anamnestic LPS-sensitization was shifted to the Th2 by the Ni-ion-immunization only once, while that of the mice without the LPS pre-treatment was not changed (Fig. 3a). On the other hand, the Th1/Th2 balance with the LPS pre-treatment was significantly shifted to the Th1 by the two Ni injections (Fig. 3b).
DISCUSSION
In this report, we showed first that anamnestic pre-treatment by LPS even predated 3 weeks effectively enhanced Ni allergy. However, we also revealed that a single treatment by Ni after the pre-treatment of LPS or repeated treatments by Ni without LPS treatment rather reduced the IFN-γ-production of spleen cells. Ni or Ni compounds conferred toxicological effects on the immune system [22]. Several immune cells including T lymphocytes are susceptible to Ni [23]. Dietary intake of NiCl2 is rerated to reduce cytokine expression of intestinal mucosa [24]. Therefore, the reduced IFN-γ-production in our observation may be caused by the toxicity of Ni. Allergic reaction may confer IFN-γ-producing capability by surpassing the toxicological effects of Ni.
LPS stimulates innate immunity via TLR 4 or 2, modifies adaptive immunity [25, 26], and also activates dendritic cells [27]. As already mentioned, Ni can also bind to TLR4 to induce nickel sensitization [14]. Our results may demonstrate that the accumulated immune memory by LPS shares a common pathway with the action mechanism of Ni, and also suggest that not only a present illness but also an anamnestic history of oral infectious disease could cause Ni hypersensitivity.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported in part by the Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (26463055). The authors declare that there are no conflicts of interest. The authors would like to express their deepest thanks to Ms. Masako Sawada for her excellent secretarial assistance.


