All published articles of this journal are available on ScienceDirect.
Are Pediatric Antibiotic Formulations Potentials Risk Factors for Dental Caries and Dental Erosion?
Abstract
Introduction:
One of the most frequent parents’ concerns is that oral antibiotic formulations induce dental damage in their children’s. This study aimed to assess the cariogenic and erosive potentials of 29 pediatric antibiotics.
Materials and Methods:
Replicates of each antibiotic were analyzed for the concentration of sugars (sucrose, glucose and fructose) and sorbitol by high performance liquid chromatography (HPLC). The pH was determined by digital pHmeter. Titratable acidity was determined in triplicate using the same pHmeter by gradual addition of 0.1N sodium hydroxide (NaOH) until pH 7.0. Viscosity measurements were carried out using a viscosimeter. In order to rank the relative performance of each medicine, the DEA (Data Envelopment Analysis) methodology was used.
Results:
Sucrose was present in most samples (n=24) with concentrations ranging from 26 to ≈ 100g% (w/w). Only one antibiotic contained sorbitol (66.9g%). Twenty seven antibiotics presented pH values ranging from 4.1 to 6.9 and most of them (n=15) showed the pH below the critical value for dissolution of hydroxyapatite. The values of titratable acidity and viscosity ranged from 0.26 to 40.48 ml and from 20 to 1780cP, respectively. DEA methodology showed that two medicines were distant from the performance frontier (Klaricid® 50mg and Zinnat® 250mg), which means that these medicines showed the worst performance and, therefore, greater potential for dissolution of dental enamel.
Conclusion:
Many antibiotics presented high concentration of sugars, high titratable acidity, pH below the critical value and high viscosity which can be considered risk factors for dental caries and erosion, when consumed frequently.
INTRODUCTION
Liquid oral medicines contain in their formulation agents to improve their appearance, bioavailability, stability, and palatability [1]. Sugars such as sucrose, fructose and glucose are added to increase bulk, palatability and, consequently, compliance [2]. These sugars are widely used because they are cheap, nonhygroscopic, and easy to process [3]. Acids are also added to medicines and commonly act as buffering agents to maintain chemical stability, control tonicity and/or to ensure physiological compatibility. In addition, they may be used to improve flavor [4]. Because of these so called “inactive ingredients”, many pediatric liquid medicines are characterized by having a high concentration of sugars, high titratable acidity and low pH. Because of these characteristics, various studies have pointed out the possible relationship between dental caries and frequent intake of liquid oral medicines [3, 5-11]. The presence of sucrose in medicines leads to pH drop of dental plaque, and also acts as substrate for fermentation of oral microbiota, [12] contributing to dental caries.
Dasanayake et al. [13] showed a higher count of Streptococcus mutans (the main microorganism involved with dental caries) in children who took antibiotics during the period considered the most critical to acquire these bacteria, which is between 1 ½ to 3 years of age, when compared to children who did not take antibiotics in the same period. Moreover, these authors observed a correlation between an increase of S. mutans colonies and caries in these individuals.
Low endogenous pH and high titratable acidity of these medicines also may favor dental erosion [4, 9, 11, 13-19], especially when the contact of the medicine with the tooth surface remains for a very long time [14]. Besides, previous in vitro studies have also shown that acidic medicines could reduce deciduous enamel hardness [13, 16] and cause morphological enamel surface alterations [18] and surface degradation of restorative materials [20]. Other concerning factors with regard to dental caries and dental erosion are the high viscosity of liquid oral medication [9, 21], lack of oral hygiene after medicine’s intake [21] and its frequency of intake, especially in children that present chronic diseases and need to make regular use of liquid oral medicines [3, 9]
Considering that some medicines may be a threat to oral health causing dental caries and erosion, the aim of this in vitro study was to investigate some physico-chemical properties of liquid oral antibiotics, such as pH, titratable acidity, sugar concentration and viscosity, which may contribute significantly to their cariogenic and erosive potential.
MATERIALS AND METHODS
Twenty nine liquid antibiotics (Table 1) available in the Brazilian market were selected for this study from a reference list registered in the National Health Surveillance Agency. All of them were oral suspensions and 23 of them had their powders reconstituted with filtered water as recommended by the manufacturers. The labels of each medicine were examined in order to gather information on sugar and acid contents.
Sugar Content Analysis
Analysis of sugars (sucrose, fructose and glucose) and sorbitol were performed using normal-phase HPLC-IR (High-Performance Liquid Cromatography coupled to a Refration Index) [11, 22]. One gram of each bottle was diluted to 50 mL in a volumetric flask with MilliQ water (Milipore, USA). The mixture was vigorously shaken and one aliquot of the supernatant (500 µL each) was diluted with pure acetronitrile in the proportion 1:1 (v/v). The mixture was then centrifuged for 2 minutes (Beckman Microfuge ETM, USA) and the supernatant was directly used for chromatography.
The concentrations were calculated using the peak heights of commercial compounds standards of sucrose, fructose, glucose (Sigma Aldrich, USA) and sorbitol (Vetec Química, Brazil). Results were average of replicates from each bottle (sugars and sorbitol concentrations - g/100g - %).
| Brand names | Manufacturers | Active principles |
|---|---|---|
| 1. Amoxil® 125 mg/5mL | GlaxoSmithKline (Mexico) | Amoxicillin |
| 2. Amoxil® 250 mg/5mL | GlaxoSmithKline (Mexico) | Amoxicillin |
| 3. Amoxil® 500 mg/5mL | GlaxoSmithKline (Mexico) | Amoxicillin |
| 4. Amoxil BD® 200 mg/5mL | GlaxoSmithKline (Mexico) | Amoxicillin |
| 5. Amoxil BD® 400 mg/5mL | GlaxoSmithKline (Mexico) | Amoxicillin |
| 6. Ampicilina® 250 mg/5mL | EMS (S. B. do Campo, SP, Brazil) | Ampicillin |
| 7. Bactrim® (200+40)mg/5mL | Roche (Rio de Janeiro, RJ, Brazil) | Sulfamethoxazole + Trimethoprim |
| 8. Bactrim F® (400+80)mg/5mL | Roche (Rio de Janeiro, RJ, Brazil) | Sulfamethoxazole + Trimethoprim |
| 9. Ceclor® 250 mg/5mL | Sigma Pharma (Hortolândia, SP, Brazil) | Cefaclor |
| 10. Ceclor® 375 mg/5mL | Sigma Pharma (Hortolândia, SP, Brazil) | Cefaclor |
| 11. Cefamox® 250 mg/5mL | Bristol-Myers Squibb (Barceloneta, Porto Rico) | Cefadroxil |
| 12. Cefamox® 500 mg/5mL | Bristol-Myers Squibb (Barceloneta, Porto Rico) | Cefadroxil |
| 13. Cefzil® 250 mg | Bristol-Myers Squibb (São Paulo, SP, Brazil) | Cefprozil |
| 14. Clavulin® (125mg+31,25)mg/5mL | GlaxoSmithKline (Mexico) | Amoxicillin + Potassium Clavulanate |
| 15.Clavulin® (250+62,5)mg/5mL | GlaxoSmithKline (Mexico) | Amoxicillin + Potassium Clavulanate |
| 16. Clavulin BD® (200+28,5)mg/5mL | GlaxoSmithKline (Rio de Janeiro, RJ, Brazil) | Amoxicillin + Potassium Clavulanate |
| 17. Clavulin BD® (400 mg+57)mg/5mL | GlaxoSmithKline (Worthing, England) | Amoxicillin + Potassium Clavulanate |
| 18. Clavulin® ES (600+42,9)mg/5mL | GlaxoSmithKline (Col Romero de Terreros, México) | Amoxicillin + Potassium Clavulanate |
| 19. Eritrex® 125 mg/5mL | Aché (Guarulhos, SP, Brazil) | Erythromycin |
| 20. Eritrex® 250 mg/5mL | Aché (Guarulhos, SP, Brazil) | Erythromycin |
| 21. Keflex® 250 mg/5mL | Lilly (São Paulo, SP, Brazil) | Cephalexin |
| 22. Keflex® 500 mg/5mL | Lilly (São Paulo, SP, Brazil) | Cephalexin |
| 23. Klaricid® 25 mg/mL | Abbott (Brazil) | Clarithromycin |
| 24. Klaricid® 50 mg/mL | Abbott (Brazil) | Clarithromycin |
| 25. Pen-ve-oral® 80.000UI/mL | Eurofarma (São Paulo, SP, Brazil) | Potassic Phenoxymethylpenicillin |
| 26. Unasyn® 250 mg/5mL | Pfizer (Latina, Italy) | Sultamicillin tosylate |
| 27. Zinnat® 250 mg/5mL | GlaxoSmithKline (England) | Cefuroxime Axetil |
| 28. Zitromax® 600 mg | Pfizer (Latina, Italy) | Azithromycin |
| 29. Zitromax® 900 mg | Pfizer (Latina, Italy) | Azithromycin |
Analysis of pH and Titratable Acidity
Antibiotics’ pH was determined at room temperature with an electrode connected to a digital pHmeter (Digimed DM 20, USA). The electrode was calibrated at the start of each session using standard buffers of pH 4.01 and 6.86 (Quimis, Diadema, SP, Brazil). Titratable acidity was measured in triplicate for each antibiotic by using the same pHmeter and increments of 0.1N sodium hydroxide (NaOH) were titrated until neutrality (pH 7.0) was reached [11] A correction factor of 0.87 was obtained by factorizing 0.1 N NaOH solution with potassium biphthalate. The total volume of 0.1 N NaOH solution required to neutralize medicines multiplicated to correction factor of 0.87 corresponded to the titratable acidity value.
Viscosity
Viscosity measurements were carried out on a AR 2000 rheometer (TA instrument, USA) using a parallel plate geometry of 25 mm diameter, at a shear rate of 0.1 to 100 s-1 and temperature of 40oC. Viscosity values were obtained at 20s-1.
| Antibiotics | Sucrosea | Sorbitola | pH | Vol NaOH (mL)b | Viscosity (cP)c |
Label content (sweeteners) |
Label content (acids) |
|---|---|---|---|---|---|---|---|
| 1. Amoxil® 125 mg/5mL | 49.97 ±1.64 | ---- | 5.71 ±0.02 | 1.38±0.05 | 30 | Sucrose | ---- |
| 2. Amoxil® 250 mg/5mL | 48.59 ±2.01 | ---- | 5.72 ±0.01 | 1.55±0.04 | 30 | Sucrose | ---- |
| 3. Amoxil® 500 mg/5mL | 36.77 ±2.46 | ---- | 5.74 ±0.01 | 2.23±0.10 | 90 | Sucrose | ---- |
| 4. Amoxil BD® 200 mg/5mL | 48.41 ±1.31 | ---- | 6.67 ±0.04 | 0.85 ±0.05 | 290 | Sugar | ---- |
| 5. Amoxil BD® 400 mg/5mL | 44.53 ±4.06 | ---- | 6.85 ±0.03 | 0.67 ±0.10 | 550 | Sugar | ---- |
| 6. Ampicilina® 250 mg/5mL | 36.43 ±1.9 | ---- | 6.16 ±0.04 | 2.73±0.00 | 40 | Sodium cyclamate, Sodium saccharin, Sucrose | ---- |
| 7. Bactrim® (200+40)mg/5mL | 55.02 ±0.14 | ---- | 6.10 ±0.01 | 2.90 ±0.09 | 1350 | Sugar | ---- |
| 8. Bactrim F® (400+80)mg/5mL | 59.02 ±8.01 | 66.90 | 5.69 ±0.02 | 4.55 ±0.22 | 1340 | Sorbitol, Sodium saccharin | ---- |
| 9. Ceclor® 250 mg/5mL | 58.86 ±0.26 | ---- | 4.34 ±0.02 | 3.78 ±0.26 | 300 | Sucrose | ---- |
| 10. Ceclor® 375 mg/5mL | 62.63 ±7.47 | ---- | 4.31 ±0.28 | 8.21 ±1.34 | 550 | Sucrose | ---- |
| 11. Cefamox® 250 mg/5mL | 58.03 ±4.41 | ---- | 5.12 ±0.02 | 2.76 ±0.13 | 20 | Sugar | ---- |
| 12. Cefamox® 500 mg/5mL | 61.01 ±2.70 | ---- | 4.86 ±0.06 | 5.54 ±0.53 | 180 | Sucrose | ---- |
| 13. Cefzil® 250 mg | 51.18 ±0.32 | ---- | 5.05 ±0.02 | 2.49 ±0.05 | 30 | Sucrose, Aspartame | Citric acid |
| 14. Clavulin® (125mg+31,25)mg/5mL | ND | ---- | 4.32 ±0.01 | 1.23 ±0.09 | 170 | Aspartame | Clavulanic acid, Succinic acid |
| 15.Clavulin® (250+62,5)mg/5mL | ND | ---- | 4.57 ±0.03 | 1.26 ±0.10 | 230 | Aspartame | Clavulanic acid, Succinic acid |
| 16. Clavulin BD® (200+28,5)mg/5mL | ND | ---- | 4.10 ±0.00 | 0.97 ±0.00 | 120 | Aspartame | Clavulanic acid, Succinic acid |
| 17. Clavulin BD® (400 mg+57)mg/5mL | ND | ---- | 4.45 ±0.01 | 1.20 ±0.05 | 190 | Aspartame | Clavulanic acid, Succinic acid |
| 18. Clavulin® ES (600+42,9)mg/5mL | ND | ---- | 4.94 ±0.02 | 0.99 ±0.10 | 340 | Aspartame | Clavulanic acid |
| 19. Eritrex® 125 mg/5mL | 68.53 ±1.03 | ---- | 5.33 ±0.00 | 6.83 ±0.22 | 530 | Sugar | Citric acid |
| 20. Eritrex® 250 mg/5mL | 74.54 ±0.02 | ---- | 5.42 ±0.02 | 1.88 ±0.05 | 270 | Sugar | Citric acid |
| 21. Keflex® 250 mg/5mL | 25.95 ±2.05 | ---- | 6.62 ±0.11 | 0.26 ±0,00 | 230 | Sucrose | ---- |
| 22. Keflex® 500 mg/5mL | 43.39 ±5.54 | ---- | 6.95 ±0.28 | 0.67 ±0,05 | 370 | Sucrose | ---- |
| 23. Klaricid® 25 mg/mL | 36.13 ±0.07 | ---- | 5.13 ±0.05 | 24.64 ±1.76 | 260 | Sugar | Citric acid |
| 24. Klaricid® 50 mg/mL | 77.46 ±10.84 | ---- | 5.04 ±0.10 | 40.48 ±0.88 | 1660 | Sugar | Citric acid |
| 25. Pen-ve-oral® 80.000UI/mL | ≈ 100 | ---- | 5.94 ±0.01 | 0.26±0.00 | 170 | Sugar, Sodium saccharin | Citric acid |
| 26. Unasyn® 250 mg/5mL | 88.81 ± 0.75 | ---- | 6.58 ±0.01 | 1.67±0.09 | 80 | Sucrose | ---- |
| 27. Zinnat® 250 mg/5mL | 89.48 ±4.89 | ---- | 4.79 ±0.00 | 12.54±2.86 | 1780 | Sucrose, aspartame | Estearic acid |
| 28. Zitromax® 600 mg | ≈ 100 | ---- | 9.75±0.00 | ------- | 1130 | Sucrose | ---- |
| 29. Zitromax® 900 mg | ≈ 100 | ---- | 10.08±0.00 | ------- | 420 | Sucrose | ---- |
Data Analysis
In order to verify how was the performance of each antibiotic in comparison with the others, the DEA (Data Envelopment Analysis) methodology has been used. DEA is a mathematical programming methodology which evaluates the relative performance of list of units with multiple attributes. It identifies a subset of performance “best-practice” elements (benchmarks) and for remaining ones, the magnitude of their lack of performance is measured by comparing to a piecewise frontier constructed from the best elements, facilitating the interpretation of the results. It allowed the evaluation of the relative performance of each antibiotic taking into account the undesirable characteristics (high concentration of sugars, low pH, high titratable acidity and high viscosity) and the desirable ones (absences of sugars, high pH, low titratable acidity and low viscosity) in the sense of tooth dissolution potential. In this analysis, the medicines with performance of 100% are those which present better characteristics in comparison with the others and, therefore, were less likely to harm the tooth enamel. On the other hand, medicines with lower percentile values are those with the worst performance and, in this way, have greater potential for dissolution of dental enamel.
| Medicines | Performance |
|---|---|
| Clavulin ES® 600 mg, Amoxil® 125 mg, Keflex® 250 mg, Ampicilina® 125 mg, Clavulin® BD 200 mg, Zitromax® 900 mg | 100% |
| Amoxil® 250 mg, Clavulin® BD 400 mg | 98% |
| Clavulin® 250 mg, Clavulin® 125 mg, Cefamox® 250 mg | 95% |
| Zitromax® 600 mg | 94% |
| Unasyn®,Pen-ve-oral® | 89% |
| Cefzil® 250 mg | 86% |
| Amoxil® 500 mg | 84% |
| Keflex® 500 mg | 62% |
| Amoxil® BD 200 mg | 56% |
| Amoxil® BD 400 mg | 55% |
| Eritrex® 250 mg | 39% |
| Cefamox® 500 mg | 34% |
| Klaricid® 25 mg | 32% |
| Bactrim® 200 mg | 26% |
| Ceclor® 250 mg | 24% |
| Eritrex® 125 mg | 20% |
| Bactrim® F 400 mg | 19% |
| Ceclor® 375 mg | 13% |
| Klaricid® 50 mg, Zinnat® 250 mg | 8% |
The BCC model with input orientation and weight constraints was applied. This analysis was carried out with the software IDEAL (Interactive Data Envelopment Analysis Laboratory), developed by COPPE/UFRJ, which allows the three-dimensional visualization of the performance frontier [23].
Pearson’s correlation coefficients were used to determine whether there was any association among concentrations of sucrose, pH, titratable acidity or viscosity, using the SPSS 20.0® software at a significance level of 5%.
RESULTS
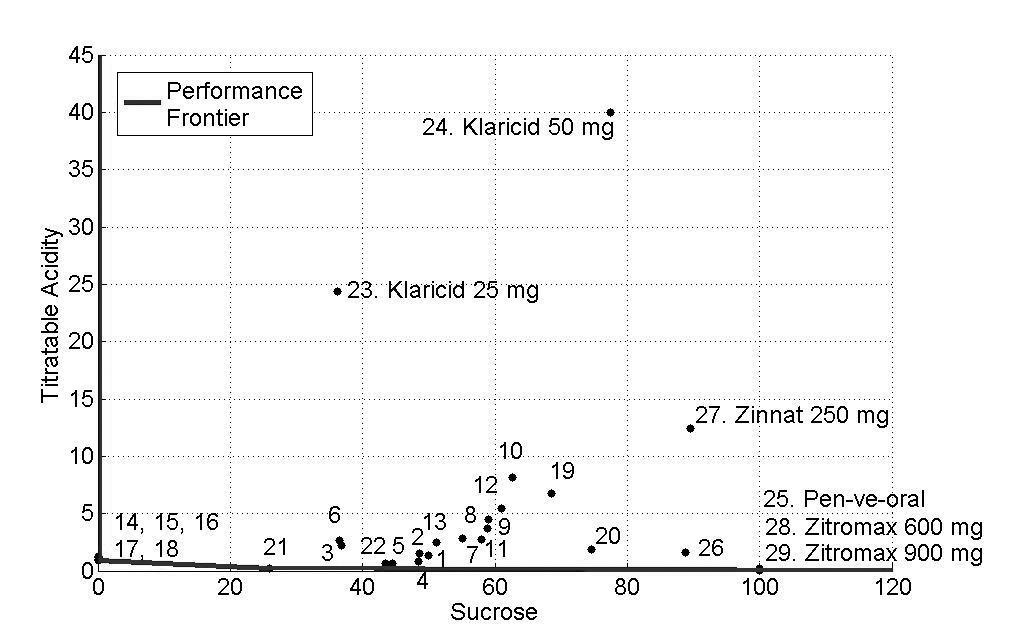
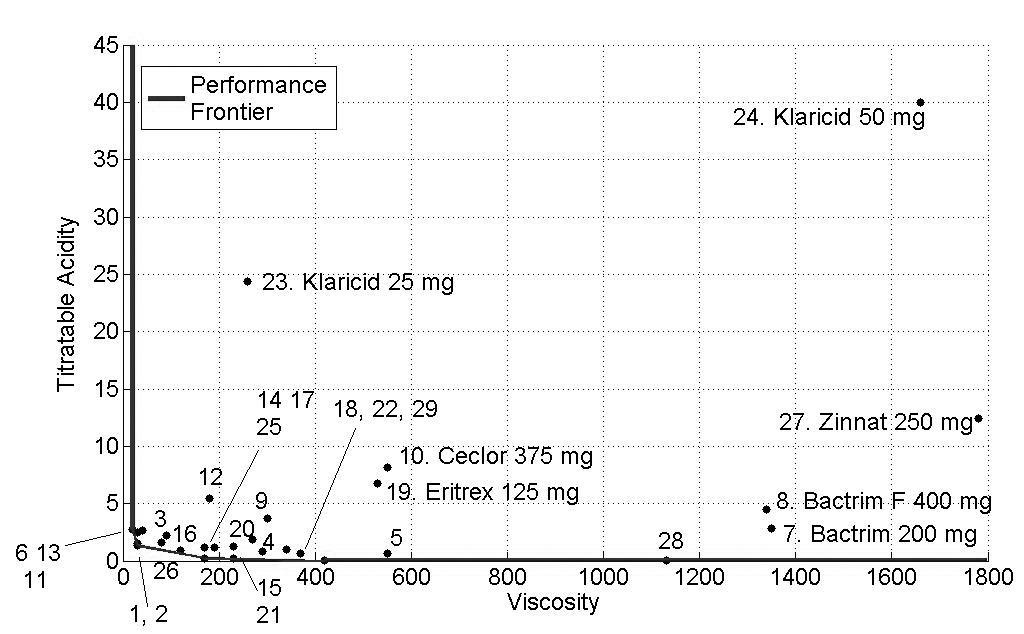
The concentrations of sucrose, fructose, glucose and sorbitol as well as pH, titratable acidity and viscosity of all antibiotics are presented as mean values in Tables 2 and 3 and Figs. (1-3) show the performance of the 29 antibiotics analyzed.
Sugar Content
Out of the 29 formulations, sucrose was present in 24 of them (83%), with concentrations ranging from 26g% to about 100g%. However, according to the medicine’s labels, only fifteen antibiotics (52%) presented sugars and only four of them specified sugar concentration. None of the antibiotics contained glucose and fructose. Only five antibiotics (17%) were sugar-free. Sorbitol was present in only one medicine (3%), with concentration of 66.9g% and associated with sucrose (Table. 2).
pH, Titratable Acidity and Viscosity
Two antibiotics (11%) presented basic pH values of 9.7 and 10.8, while the others presented pH values ranging from 4.1 to 6.9. Fifteen of the 29 analyzed antibiotics (52%) presented pH below the critical value for dissolution of hydroxyapatite (pH 5.5). Values of titratable acidity and viscosity ranged from 0.26 to 40.48 mL and from 20 to 1780 cP, respectively. Pearson’s correlation coefficient’s showed that there was no correlation between pH and titratable acidity (p>0.05) neither between sugar content and viscosity (p>0.05).
Performance of Medicines (Software IDEAL)
The DEA methodology allowed the simultaneous evaluation of the analyzed variables (concentration of sugars, pH, titratable acidity and viscosity) and from the scores obtained, antibiotics were classified according to their relative performance.
In order to facilitate the interpretation of the results, three figures of the performance frontier were plotted (Figs. 1-3). Each medicine was placed on its corresponding point, according to its attributes, and can be identified by its corresponding number. Medicines placed in the vertex points of each face are considered as references or benchmarks, have 100% performance and constitute the performance frontier.
Fig. (1), shows the performance frontier for sucrose and titratable acidty. It can be observed that medicines 14 (Clavulin® 125mg), 15 (Clavulin® 250mg), 16 (Clavulin® BD 200mg), 17 (Clavulin® BD 400mg) and 18 (Clavulin ES® 600mg) presented low levels of sucrose and titratable acidity and, for this reason, are close to the frontier. The medicines 25 (Pen-ve-oral®), 28 (Zitromax® 600mg) and 29 (Zitromax®900mg), despite of being close to the performance frontier due to low titratable acidity, have high concentration of sucrose, which is an undesirable characteristic. On the other hand, the medicines 23 (Klaricid® 25mg/5mL), 24 (Klaricid® 50mg/5mL) and 27 (Zinnat® 250mg/5mL) presented the worst performance because of their high levels of sucrose and titratable acidity.
Fig. (2) identifies the medicines with high levels of viscosity and titratable acidity. Despite of their high viscosity, the medicines 7 (Bactrim® 200mg/mL), 8 (Bactrim F® 400mg/5mL) and 27 (Zinnat® 250mg/5mL) showed low levels of titratable acidity. Medicines with low viscosity and titratable acidity and close to the performance frontier presented a better performance. Again, Klaricid® 50mg/mL was distant from performance frontier because of their high levels of titratable acidity and sacarose concentration, presenting the worst performance (8%).
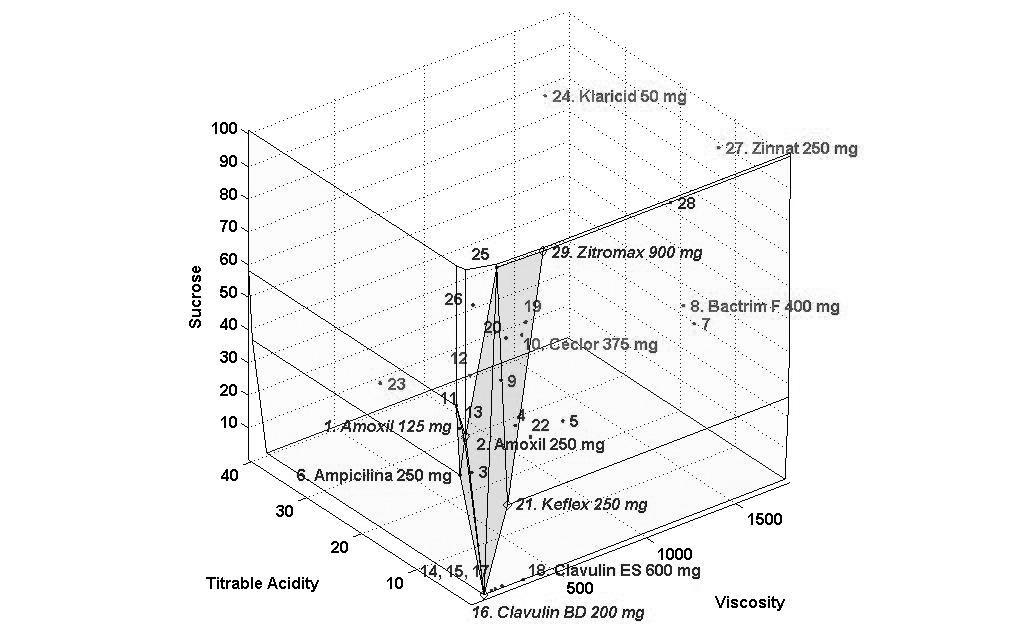
Finally, Fig. (3) shows the 3D visualization of the performance frontier and identifies the best and worst medicines see also Table 3. Regions with higher density of medicines show a group of medicines with an average performance. Klaricid® 50 mg/5mL and Zinnat® 250 mg/5mL were distant from the performance frontier, which means that these medicines showed the worst performance and, therefore, greater potential for dissolution of dental enamel.
DISCUSSION
Antibiotics, which were chosen for this study, as well as cough medicines are the most common sugar-containing medicines regularly used by young children [24]. Considering that they may still be used over relatively long periods, it can be a concern for oral health. Moreover, these medicines can present erosive potential due to presence of acids in their formulations, low pH, high titratable acidity, presence of buffering agents and absence or low concentrations of ions including those of calcium, fluoride, and phosphate in their composition [9, 13, 25, 26].
Although the antibiotics analyzed in this study did not contain glucose and fructose, the presence of high sucrose concentration in 83% of the formulations, ranging from 26g% to about 100g%, poses a real problem to the young consumers. It may increase the caries risk, since sucrose is considered the most cariogenic dietary carbohydrate, because it is fermentable, and also serves as a substrate for the synthesis of extracellular and intracellular polysaccharides in dental plaque [27, 28]. Others studies have reported a predominant use of sucrose as a sweetener in medicines [11, 29-31]. It should be emphasized that in this study three antibiotics presented concentrations of sucrose with values close to 100g%. This peculiarity may be explained mainly by the fact that as sugar is denser than water, then, 100g of the sugar will occupy less space than water. Additionaly, the concentrations of the bioactive compound of these oral suspensions are expressed in µg%, which is under the detection limit of the equipment. This high sugar concentration is unacceptable considering its cariogenic potential. On the other hand, there are controversial studies that show that despite of the potentially high sucrose concentration in antibiotics, they work by reducing the number of Streptococcus mutans and therefore are anticariogenic and may counteract sucrose´s effect [32, 33].
All sweetening agents identified in this study were in accordance with the medicines’ labels. However, sugar concentrations were unspecified. In relation to sugar-based medicines, most labels (93%) alerted that the product should not be consumed by diabetic patients; however, none of them mentioned its cariogenic potential, especially when used for long periods of time.
Other factors analyzed in the present study were pH and titratable acidity, and it is still generally accepted that titratable acidity is a better indicator of erosive potential than pH alone [34, 35]. Titratable acidity is the property of an acid solution of keeping its pH when neutralizing agents are added, that is, the measure of its buffering capacity. In this way, a substance with low titratable acidity is readily neutralized by oral fluids, the opposite occurs with that characterized by high acidity, which causes prolonged drop in pH and higher demineralization of the tooth structure. [34, 36] According Jensdottir et al. [37] teeth exposed to a limited volume of a drink with low pH and high titratable acidity for a long time will result in erosive potential and both qualities combined will result in the highest erosive potential. The liquid oral antibiotics analyzed in this study had titratable acidic values ranging from 0.26 to 40.48 mL, but some of them proved to be discrepant in relation to others, which can cause concern. It is now widely accepted that the total titratable acidity is a more accurate measure of the total acid content of a drink, and may, therefore, be a more realistic means of predicting erosive potential. Although the mean endogenous pH for Klaricid® 50mg/5mL and Klaricid® 25mg/5mL was 5.04 and 5.13, their mean titratable acidity was high at 40.48 and 24.64 mL, respectively. It reflects the incorporation of acid in excess in their formulation, which according medicine’s labels is citric acid.
Citric acid is considered the main acid used in prolonged oral clearance medicines and as such, is a weak acid, dissociated in solutions with a higher pH and able to act as a buffer over a range of pHs. However, it is a potent erosive agent because of its ability to chelate calcium in hydroxyapatite, thus increasing enamel’s rate of dissolution on exposure to the acid [38]. In the present study, six of the twelve medicines which specified presence of acids presented citric acid in their composition. Other acids presents were clavulanic acid, succinic acid and estearic acid. Again, just as the sweeteners, the real amount present in these products were not stated on the labels.
On the other hand, it is important to mention that the consumption of an acidic drink will stimulate salivary flow [39]. It is possible, therefore, that a more acidic drink may be either cleared from the mouth or neutralized more rapidly, due to the increased salivary washing action and buffering capacity, and so will spend less time in contact with the teeth. Clearance of a drink from the mouth will also depend on the ability of a drink to adhere to the enamel [40].
It is necessary to highlight the real impact of the use of oral liquid antibiotics in daily life of children. The amoxicillin, with or without association of clavulanate, is a medicine widely used by children in the treatment of infections. According to our study, despite of presenting a better performance than a lot of medicines (Table 3), the group of amoxicillin (medicines 1 - 5) presented pH values between 5.71 to 6.85 and high concentration of sugar (36.77 to 49.97) which were worrying in relation to oral health. This fact was also noted in medicines 14-18 that showed lower pH values ranging from 4.10 to 4.94 despite of being sugar free, which can favor to dental erosion. Other medicines widely used in routine of immunocompromised patients to prevent opportunistic infections are Bactrim® and Bactrim F® (medicines 7 and 8). These medicines appeared distant from the performance frontier because of their high concentrations of sucrose and high viscosity (Fig. 3), despite of their low titratable acidity (Fig. 2).
It is also important to note the main factors that seem to increase the likelihood of an individual suffering from dental erosion includes salivary flow rate, pH, buffering capacity and pellicle formation [41-43]. All these factors added to the properties of the drink itself, as well as associated factors relating to the method of drinking, frequency of consumption, salivary parameters and dental plaque play a role in the development of dental erosion. In addition, in medically compromised children, many of these medicines may be taken at times remote from food intakes or at night when salivary flow is reduced, and these factors may add to the potential erosive challenge [4].
This fact is of concern because of the known relationship between carbohydrates and dental caries, and it becomes even more alarming if one considers that not all the pediatricians gave parents instructions about oral hygiene after medicine’s intake. In a previous study, [44] pediatricians did not perceive the correct relationship between the presence of acidity in medicines and dental erosion; however, most of them presented a reasonable awareness about the relationship between sugared pediatric medicines and dental caries. Nevertheless, not all of them recommended oral hygiene after their consumption (50.80%). Another study, verified that only 21.2% of the guardians that associated the use of pediatric liquid medicines with the development of dental caries performed the oral hygiene of their children. In addition, 84.9% of those guardians had never received instructions of oral hygiene after the intake of medicines [45].
In relation to oral hygiene immediately after acidic products, literature has reported that eroded enamel is more susceptible to wear by tooth-brushing and to toothpaste abrasion [46, 47]. In this way, after consuming acidic foods or drinks, tooth-brushing should be delayed to allow the saliva to exert its natural remineralizing action on the eroded enamel, thereby resulting in increased resistance to abrasion [47]. Recommendation on immediate water rinse and delayed tooth-brushing after syrup medicines’ ingestion could be proposed at the time of prescription. Medicine’s labels also should alert in relation to the possibility of cause dental caries and erosion because many products could be consumed for prolonged periods, several times a day, at bedtime, and without adequate oral hygiene, will certainly contribute to dental caries and erosion.
CONCLUSION
Most of the analyzed antibiotics presented high sugar concentration, titratable acidity and viscosity, and low pH which can be considered as risk factors for dental caries and erosion when consumed frequently. We advise, therefore, the use a warning about the medicine’s cariogenic potential in their labels. It is also necessary to recommend oral hygiene after the medicine’s intake. Clinical studies are necessary to determine the real extension of the problem.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors are grateful to Prof. Elizabete Lucas and Léa Lopes for their support in the viscosity analysis at IMA (Instituto de Macromoléculas Eloisa Mano), Federal University of Rio de Janeiro (UFRJ) and to Viviane Pierro for her critical review of this manuscript. The authors also would like to thank CNPq for the research grant (308029/2006-2) and FAPERJ (E-26/171.241/2006) for the financial support.


