All published articles of this journal are available on ScienceDirect.
Effect of Different Desensitizing Protocols on Pulp Inflammatory Responses in Whitened Teeth: A Randomized Clinical Trial
Abstract
Purpose:
This randomized controlled, blind clinical trial evaluated the efficacy of different desensitizing protocols in preventing pulp inflammation after whitening treatment with hydrogen peroxide (HP) at 35% (Whiteness HP 35%).
Materials and Methods:
Thirty healthy third human molars extracted by orthodontic indication were randomized and allocated into five groups (n=5): NC (negative control): without intervention; PC (positive control): HP; PBM: HP + photobiomodulation with a Watts LASER; CPP: HP + casein phosphopeptide amorphous calcium phosphopeptide (CPP-ACP); and NANO: HP + nano-hydroxyapatite. The in-office whitening was performed in two sessions with a single 45 minutes application at an interval of 48 hours. Pulp tissues were extirpated for immunohistochemical analysis. Immunoreaction for activated caspase-3 was observed, and images were acquired using an Axio Scope A1 microscope. The Kruskal-Wallis test was used to evaluate the immunoexpression of caspase-3.
Results:
Comparisons between the PC and NC groups revealed a statistically significant difference (p<0.05) for the analysis of caspase-3 immunoexpression. A statistically significant difference (p<0.05) was also observed for the CPP and PBM groups in relation to the PC.
Conclusion:
Photobiomodulation and CPP-ACP are promising alternatives to minimize pulpal inflammation induced by tooth whitening.
Clinical Trial Registration Number:
NCT04548674.
1. INTRODUCTION
In-office tooth whitening is a conservative and effective aesthetic treatment protocol that fulfills patients' desires for a whiter smile [1]. However, the use of hydrogen peroxide (HP) in high concentrations can result in side effects in dental tissues [2]. The active concentration of HP ranges from 20% to 40%[3]. Its high concentration can trigger potential pulp damage and cause a reversible inflammatory condition [4], as it is a considerable cytotoxic irritant to pulp tissue [5], causing dental sensitivity reported by patients as sharp and acute pain.
Tooth sensitivity during and after whitening has been associated with the fast entry of the whitening agent through the tooth surface until it reaches the pulp [6]. Contact with the aggressive agent can lead to an inflammatory reaction, characterized by the release of cell-derived factors, such as adenosine triphosphate (ATP) [5], neuropeptides and prostaglandins, which in turn sensitize the pulp nociceptors, allowing the perception of pain by the patient [7]. Additionally, the inflammatory process is usually associated with vasodilatation and increased pulpal blood flow [8]. Some desensitizing therapies have been used to minimize post-whitening sensitivity [9].
Photobiomodulation (PMB) is a desensitizing therapy with the capacity to promote the regeneration of injured tissues, in addition to analgesic, anti-inflammatory, and biomodulatory properties [10], with effective action in the control of dental sensitivity [11].
Amorphous calcium phosphopeptide-phosphate casein nanocomplexes (CPP-ACP) acts as a calcium and phosphate reservoir when incorporated into the dental plaque and tooth surfaces [12]. It acts to reduce demineralization and promote remineralization in the tooth enamel [13].
Nano-hydroxyapatite (NANO) is a biocompatible material widely used in dental remineralization [14]. Its use in clinical practice as desensitizing therapy has demonstrated great efficacy [15].
Caspases are cysteine proteases that are essential in programmed cell death and inflammation [5]. Among them, caspase-3 is an apoptotic executor that, after activation by caspase-8 or -9, cleaves many other functionally critical proteins inside the cell, leading to apoptosis [6]. Many therapies, including cytotoxic drugs, radiotherapy, or immunotherapy, can cause cell death by caspase-3 activation [16]. This protease is present in the cells of various tissues of the human body, including the dental pulp cells, and its high immunoexpression can flag an inflammatory process [4], with consequent pain, acting as an indicator of extrinsic aggression to the cell that leads to apoptosis [17] Thus, the activation of caspase-3 can be used as a marker to control a treatment that harms these cells, such as the whitening treatment.
This randomized clinical trial evaluated the efficacy of different desensitizing therapies in preventing pulp inflammation after a whitening treatment with HP at 35%. According to the authors’ best knowledge, no previous clinical trial has evaluated the potential of these desensitizing protocols in preventing pulp inflammation. The following null hypothesis was tested: H01 - there is no difference in protein immunoex- pression between the treatment groups (photobiomodulation, HP + casein phosphopeptide amorphous calcium phospho- peptide (CPP-ACP), and nano-hydroxyapatite) in relation to the controls after tooth whitening.
2. MATERIALS AND METHODS
2.1. Ethical Aspects
This clinical trial followed the recommendations described by the Consolidated Standards of Reporting Trials [18]. The research ethics committee of the Oncological Research Center of the Federal University of Pará reviewed and approved the trial under number 3.507.272. This study was registered in the Clinical Trial Registry (http://www.ClinicalTrials.gov) under the protocol (NCT04548674). According to the Declaration of Helsinki, all research participants signed an informed consent form [19].
2.2. Study Design
This study was a randomized blind clinical trial conducted at the Federal University of Pará from February 2021 to December 2021.
2.3. Inclusion and Exclusion Criteria
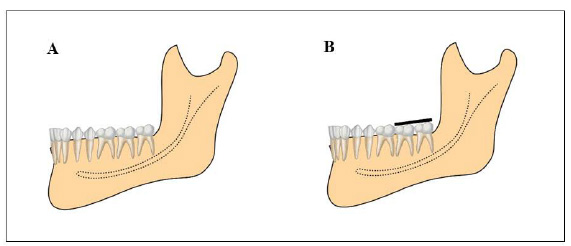
All participants included in this study were recruited using advertisements placed on notice boards located in the university buildings and were examined and selected based on the following inclusion criteria: age 20–26 years; lower third molars class I position A (Fig. 1); upper third molars in position A (third molar occlusally at the same level or below the neighbouring tooth) and horizontal (the long axis of the upper third molar perpendicular to the upper second molar), according to the classification by Pell & Gregory [20]; absence of caries and enamel fracture; absence of periodontal disease; extraction of third molars for orthodontic reasons; and no allergy to the anaesthetic. The exclusion criteria were calcification in the middle third of the root; drug users or smokers; previous whitening; root apex with incomplete formation, and systemic problems that would render the surgery non-feasible.
| Groups (n=30) | Whitening Treatment | Desensitizing Treatment |
|---|---|---|
|
NC (Negative Control) |
__________ | __________ |
|
PC (Positive Control) |
Whiteness HP 35% (FGM, Joinville, SC, Brazil) |
__________ |
|
PMB (Photobiomodulation Group) |
Application of LASER - Photon lase III (DMC Equipment, São Carlos, SP, Brazil) | |
|
CPP (CPP-ACP Group) |
CPP-ACPF application - MI paste plus (GC America, Recaldent ®, Alsip, IL, USA) | |
|
NANO (Nanohydroxyapatite Group) |
Application of Nanohydroxyapatite - Desensitize Nano P (FGM, Joinville, SC, Brazil) |
2.4. Calculation of Sample Size
The calculation of sample size was based on a previous pilot trial using G Power 3.1 software (Heinrich-Heine-Universität, Düsseldorf, Germany). The calculation was made considering a test power of 80%, a significance level of 5%, and 20% sample loss at the end of the trial. Thus, 30 third molars were included in the randomization.
2.5. Randomization and Allocation Concealment
A researcher not involved in the intervention or evaluation generated a random list in the BioEstat 5.0 software (Civil Society, Mamirauá, Pará, Brazil). The participants were defined as a block in the randomization process, and the treatment sequence (placebo or desensitizing treatment) was randomly defined for each block using a site freely available online (www.sealed.envelope.com). The sequence was inserted in sealed envelopes, numbered from 1 to 30, which were opened by the operator only at the time of the intervention. The patients were numbered according to the enrolment sequence.
2.6. Blinding
In this blinded study, the evaluators of the histological slides for analysis of the degree of pulp inflammation and immunohistochemistry were unaware of the groups to which each slide belonged and the statistical analysis of the results. The research relied on a single operator to perform the whitening, desensitizing, and exodontia throughout the clinical phase of the study.
2.7. Study Intervention
During this 5-day clinical trial, all participants received prophylaxis on all teeth before the initiation of the study, using fluoride-free prophylactic paste (NuPro Supremo, Dentsply, Nova York, PA, USA) to remove stains and outer plaques, in addition to a kit containing fluoride-free paste, a toothbrush with soft bristles (Soft ORAL-B, Sao Paulo, Brazil), dental floss and written instructions on ways to use the material. The in-office whitening treatment was performed in two 45 minutes sessions using HP at 35% (Whiteness HP FGM, Joinville, SC, Brazil) at a 48 hours interval. A Top Dam photopolymerized resin barrier (FGM, Joinville, SC, Brazil) was applied to the gingival tissue around the third molar to be extracted. The whitening gels were mixed and applied to the vestibular surfaces with an average thickness of 1 mm. The eligible third molars (n=6) were allocated to five groups according to the corresponding desensitizing treatment in this study.
2.8. Desensitizing Treatment
Immediately after each whitening treatment session, the photobiomodulation (PBM), CPP, and nano-hydroxyapatite (NANO) groups received the corresponding desensitizing treatment described in Table 1.
2.9. Exodontia of Third Molars
The molars were extracted 48 hours after the end of the second whitening session. Before exodontia, panoramic radiographs, complete blood count, coagulation profile, and blood glucose were recorded for all volunteers. The anaesthetic used was mepivacaine HCl 2% with adrenaline 1:100.000 (Nova DFL, Jacarepaguá, RJ, Brazil). For exodontia of the upper third molars, the upper posterior alveolar nerve and the upper palate were anesthetized, while in the lower third molars, field blockade of the lower alveolar, oral, and lingual nerves was performed. A no. 9 Molt periosteal elevator (Quinelato, São Paulo, SP, Brazil) was used for exodontia of the third molars to detach the dental papillae between the teeth, together with a straight Seldin elevator (Quinelato, São Paulo, SP, Brazil) to move the root of the alveolus. Forceps 18L, 18R, and 17 (Quinelato, São Paulo, SP, Brazil) were used for the exodontia of the teeth in the left maxillary quadrant, right maxillary quadrant, and mandibular quadrants, respectively. Following this, irrigation of the surgical cavity was performed, and extraction site was approximated using x-stitch sutures with 3-0 silk (Shalon, Sertix, Brazil), which was removed after 7 days. After the surgery, oral Ibuprofen 600 mg (Glaxo Smith Kline Brazil Ltd., Rio de Janeiro, RJ, Brazil) every 8 hours for 4 days and Paracetamol 750 mg (Glaxo Smith Kline Brazil Ltd., Rio de Janeiro, RJ, Brazil) were prescribed.
2.10. Laboratory Procedures
2.10.1. Sample Preparation
Immediately after exodontia, the teeth were sectioned at the level of the cervical third of the roots using a diamond tip no. 2136 (KG Sorensen Ind. Com. Ltd., São Paulo, SP, Brazil), coupled to a refrigerated handpiece. Then, the coronary portion was immersed in a fixative solution (formaldehyde at 10% with pH 7), presenting a volume 20 times the histological specimen under analysis, where it remained for 48 hours. To remove the pulp tissue, grooves were made in the vestibular-lingual and mesial-distal directions, perpendicular to the pulp surface, with a diamond tip no. 4219 (KG Sorensen, São Paulo, SP, Brazil) at high speed (Vaz et al., 2016) [21]. To preserve the pulp tissue, the enamel/dentin fragments were removed using a mini Ochsenbein chisel (Hu-Friedy, Rio de Janeiro, RJ, Brazil). The pulp tissues were re-immersed in the fixative solution until the histological processing. This preparation of the human pulp tissue samples was performed per the methodology proposed by Vaz et al. (2016) [21].
2.10.2. Immunohistochemistry
Thirty cases of tooth pulp were formalin-fixed, and paraffin-soaked tissues were recovered for immunohis- tochemistry. Three-micron sections were obtained and mounted on StarFrost® slides (Knittel, Berlin, Germany, DE). The sections were depilated in xylene and rehydrated in graduated solutions of ethanol. The antigen was recovered in the Pascal chambers (Dako, Carpinteria, CA, USA) for 30 minutes. The sections were immersed in HP at 3% in methanol for 20 min to inhibit endogenous peroxidase activity and then blocked using bovine serum albumin at 1% (Sigma Chemical Corp., San Luis, Missouri, USA) in phosphate-buffered saline solution for 1 hour. The tissue sections were incubated with primary anti-caspase 3/CPP32 antibodies (Biosystems, Whittier, CA, USA) at a dilution of 1:150 and incubated for 1hour at room temperature. The primary antibodies were diluted in antibody diluent solution (Dako, Carpinteria, CA, USA) and the sections were incubated for 30 minutes with a polymer marked with horseradish peroxidase without biotin of the Advance Detection System (Dako, Carpinteria, CA, USA). Diaminobenzidine (Sigma Chemical Corp., San Luis, Missouri, USA) was used as the chromogen and the sections were contrasted using Mayer's hematoxylin (Sigma Chemical Corp., San Luis, Missouri, USA). Oral squamous cell carcinoma samples were used as a positive control. The replacement of specific primary antibodies with nonimmune serum served as a negative control for all the samples. This protocol was adapted per the study by Kumamoto et al. (2001) [22].
2.10.3. Evaluation by Immunostaining
Immunostaining was performed for caspase-3. Six randomly selected light field images of each sample were obtained using an Axio Scope A1 microscope (Carl Zeiss, Germany) equipped with a colour charged-couple device camera (AxioCam MRc; Carl Zeiss). All images were taken using the same magnification (40×). Areas of diamino- benzidine staining were separated and segmented using the colour deconvolution plugin, written by Gabriel Landini (Landini & Perryer, 2009) [23], in the ImageJ public domain software developed by Wayne Rasband (NIMH, NIH, Bethesda, MD, USA, http://rsbweb.nih.gov/ij/). After image segmentation, the total epithelial area was evaluated and the percentages of the stained areas in all the samples were analyzed.
2.11. Statistical Analysis
Data were analyzed using BioStat 5.3 software. For comparison between groups of caspase-3 immunoexpression, the Kruskal-Wallis Test and Dunn's post-test were conducted for non-parametric samples. The G-test was used to analyze the demographic characteristics of the participants. The signifi- cance level was 5% for all tests.
3. RESULTS
3.1. Participants
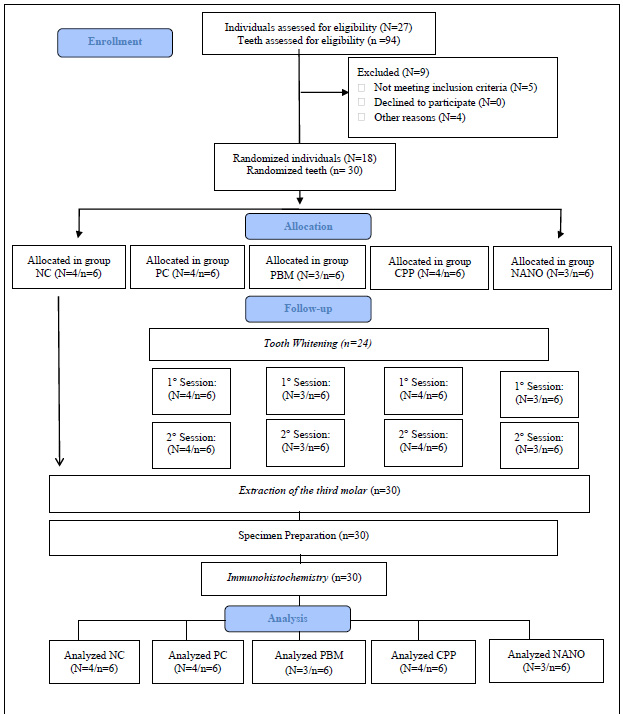
A total of 27 participants were evaluated, with 18 of them being randomized, treated, and followed up, as illustrated in Fig. (2). Thirty pulps, removed from third molars, were analyzed using immunohistochemistry.
3.2. Demographic Characteristics
The demographic characteristics of the 18 participants who completed the study are described in Table 2. In the final sample, there was a higher proportion of male participants (N=11; 61.11%) than female participants (N=7; 38.89%). The mean age of the participants was 23.44 (standard deviation=2.87; variation=20–26) years. On comparison of the different treatment groups, no significant difference was detected in any of these characteristics (p>0.05).
3.3. Caspase-3 Immunoexpression
All the samples showed caspase-3 immunoexpression, predominantly in the cytoplasm of the dental pulp cells, and nuclear expression was sometimes also observed (Fig. 3).
| Groups | NC | PC | PBM | CPP | NANO | p |
|---|---|---|---|---|---|---|
| (N=4/n=6) | (N=4/n=6) | (N=3/n=6) | (N=4/n=6) | (N=3/n=6) | ||
|
Sex
N (%) |
||||||
| Feminine | 2 (50) | 2 (50) | 1 (33.33) | 1 (25) | 1 (33.3) | 0.4792* |
| Masculine | 2 (50) | 2 (50) | 2 (66.67) | 3 (75) | 2 (66.67) | |
| Age, Years | ||||||
| Mean | 22.60 | 22.68 | 24 | 22.30 | 24.60 | 0.4251* |
| Interval | 22-25 | 20-26 | 23-25 | 20-22 | 21-26 | |
| Comparison of Groups | p-value |
|---|---|
| NC × PC | < 0.05 |
| NC × PBM | 0.731 |
| NC × CPP | 0.952 |
| NC × NANO | 0.622 |
| PC × PBM | < 0.05 |
| PC × CPP | < 0.05 |
| PC × NANO | 0.543 |
On comparison among treatments (PBM, CPP, and NANO groups) and control groups for caspase-3 immunoexpression analysis (Table 3), there was a statistically significant difference (p<0.05) between the group that received whitening only (PC) and the non-intervention group (NC); PC group and the group that received PBM and PC group and the group that received treatment with calcium phosphate and amorphous calcium phosphate (CPP). There was no statistically significant difference between the NC and treatment groups; and the PC group and the group that received treatment with NANO.

4. DISCUSSION
Clinical trials [24, 25] and in vitro studies [26-28] that investigated the effect of whitening on the dental pulp reported changes in the pulp tissue caused by the penetration of HP during whitening treatment [29].
HP penetrates the cell membrane, alters cell viability, and induces apoptosis in dental pulp cells [30] where a higher concentration of HP can cause necrosis in the pulp and prolonged effects on the apoptotic process and lower concentrations lead to inflammation, cell proliferation, and apoptosis, with reduction of these processes over time [31]. High caspase-3 immunoexpression can signal this inflammatory process, which is consequently painful for the patient soon after the procedure, acting as an indicator of extrinsic aggression to the cell that leads to apoptosis [17]. Immunohistochemical analysis was used in this study to detect caspase-3 protein, an important finding since it is the first clinical trial to evaluate this protein in human pulp in teeth bleached after different desensitization protocols. A statistically significant difference was observed for caspase-3 immunoexpression between the NC and PC groups, with higher protein expression in the PC group, treated with HP at 35% only, confirming the results by Wu et al. (2013) [32]. Caspase-3 expression in the study, therefore, represented a parameter to indicate the presence of apoptosis in the pulp cells.
Therapy using PBM with the infrared light spectrum and wavelength variation between 810 nm and 830 nm has been associated with inhibition of the proliferative rate of some cell types [33]. Studies have shown that PBM acts on cells through a main photoreceptor, cytochrome c oxidase, a terminal enzyme of the mitochondrial electron transport chain that plays a vital bioenergetic role in the cell [34, 35]. The absorption of photons by cytochrome c oxidase leads to the electronically excited state, stimulating the respiratory chain. Hence, there is an increase in the electrical potential of the membrane, and greater oxidative metabolism, resulting in the greater synthesis of ATP and reactive oxygen species (ROS) [36]. ROS resulting from the activation of the respiratory chain can have beneficial or harmful effects. According to Huang et al. (2009) [37], low levels of ROS are associated with biostimulant effects, while high levels have been related to inhibitory and cell death-inducing effects, such as those occurring in in-office tooth whitening [38]. PBM stimulates cell growth directly through regulation of the gene expression related to cell proliferation, and indirectly through regulation of genes related to migration and remodelling, DNA synthesis and repair, and cell metabolism. PBM also increases cell proliferation by suppressing cell apoptosis [39], which may explain the reduced expression of the irreversible apoptosis marker in the results of this study when compared to the PC group, leading to a rejection of the H01 hypothesis. In this study, the NC group and PBM showed a statistically significant difference from PC which, in turn, presented caspase-3 immunoexpression. This shows the efficacy of PBM therapy, which obtained results similar to those of the NC group. Benetti et al. (2017) [8] and Dantas et al. (2010) [16] showed that PBM, with specific parameters, may minimize pulp damage caused by whitening.
Casein protein, present in CPP-ACP, can stabilize the calcium and phosphate ions in the dental enamel, favouring the mineral gain in the demineralized enamel subsurfaces [40]. A comparison between the CPP and NC groups showed no difference in the immunoexpression of apoptosis markers, on the other hand it was statistically significant when compared to the PC, revealing that CPP-ACP was able to inhibit cell apoptosis triggered by HP at 35% after the whitening treatment. The immunoexpression of apoptosis markers is induced by the stress of whitening [41] in the dental pulp, triggered by the mitochondrial pathway [42]. For this route to be inhibited, an agent with direct action on the mitochondrial cells should be present inside the pulp to repair the caspase-3 expression [29]. A previous laboratory study reported the formation of a coating blanket around the enamel prisms whitened and treated with CPP-ACP and smaller space between the hydroxyapatite crystals, with greater mineral deposition on the enamel surface [43], in agreement with the results of this study, demonstrating a protective action of CPP-ACP.
Hydroxyapatite-based materials have remineralizing effects on the surface of altered enamel, capable of forming a thick and homogeneous layer of apatite covering the surface of demineralized enamel [44]. The nanometric spectrum of the hydroxyapatite particle allows the nanoparticle to continuously penetrate the enamel and occupy the sites left by the demineralized enamel crystals [45]. NANO can deposit on the surface and subsurface of the enamel [46], reducing the demineralized enamel's structural defects and the whitening agent's diffusion in dental tissues [47]. The comparison between the control and NANO groups showed no statistically significant difference for the immunoexpression of apoptosis markers, revealing that treatment with NANO was unable to inhibit the formation of apoptotic cells. The hydroxyapatite nanoparticles are similar to the hydroxyapatite crystals of tooth enamel [48], which facilitates their action on the enamel. The results of the immunoexpression of apoptosis markers in this study may be derived from the incomplete obliteration of the dentinal tubules by NANO particles, allowing the diffusion of the whitening agent in dental tissues, leading to apoptotic cell death [49]. There are no clinical trials in the scientific literature evaluating the NANO-based desensitizer with the same objective presented in this study.
Although we have not investigated the sensitivity index reported by patients for the different treatments, presenting a limitation of this study, we suggest that new studies should be conducted evaluating this variable and correlating it with the immunohistochemistry.
CONCLUSION
We can conclude that PBM and CPP-ACP are promising protocols for minimizing pulpal inflammation induced by tooth bleaching.
LIST OF ABBREVIATIONS
| HP | = Hydrogen Peroxide |
| ROS | = Reactive Oxygen Species |
| NC | = Non-intervention Group |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research ethics committee of the Oncological Research Center of the Federal University of Pará reviewed and approved the trial (Approval no.3.507.272).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written and informed consent was obtained from the participants.
STANDARDS OF REPORTING
CONSORT guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [C.M.S.], on special request.
FUNDING
This work was supported by the National Council for Scientific and Technological Development and the Federal University of Pará for granting scholarship number (161846/2018-1) for the development of the research.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
To the National Council for Scientific and Technological Development (CNPq) and the Federal University of Pará for their support in the development of research.


